Sunday, August 26, 2007
Saturday, July 28, 2007
Biochemical and genetic Characterization of a Streptomyces sps from Everest region and chemical characterization of antibiotics produced therefrom.
Biochemical and genetic Characterization of a Streptomyces sps from Everest region and chemical characterization of antibiotics produced therefrom.
Principal investigator
Jyotish Yadav
Student from eighth semester
Universal Science College
Pokhara University
Maitidevi, Kathmandu
2007
Supervisor
Upendra Thapa Shrestha
Universal Science College
Pokhara University
RLABB
Maitidevi, Kathmandu
Research Lab
Research Laboratory for AgriculturalBiotechnology and Biochemistry (RLABB)
Introduction
Actinomycetes comprise an extensive and diverse group of Gram-positive, aerobic, mycelial bacteria with high G+C nucleotide content (>55%), and play an important ecological role in soil cycle. The name of the group actinomycetes is derived from the first described anaerobic species Actinomyces bovis that causes actinomycosis, the ‘ray-fungus disease’ of cattle. They were originally considered to be intermediate group between bacteria and fungi but are now recognized as prokaryotic microorganisms (Kuster 1968).
The majority of Actinomycetes are free living, saprophytic bacteria found widely distributed in soil, water and colonizing plants. Actinomycetes population has been identified as one of the major group of soil population (Kuster 1968), which may vary with the soil type. They belong to the order Actinomycetales (Superkingdom: Bacteria, Phylum: Firmicutes, Class: Actinobacteria, Subclass: Actinobacteridae). According to Bergey's Manual Actinomycetes are divided into eight diverse families: Actinomycetaceae, Mycobacteriaceae, Actinoplanaceae, Frankiaceae, Dermatophilaceae, Nocardiaceae, Streptomycetaceae, Micromonosporaceae (Holt, 1989) and they comprise 63 genera (Nisbet and Fox, 1991). Based on 16s rRNA classification system they have recently been grouped in ten suborders: Actinomycineae, Corynebacterineae, Frankineae, Glycomycineae, Micrococineae, Micromonosporineae, Propionibacterineae, Pseudonocardineae, Streptomycineae and a large member of Streptomyces are still remained to be grouped (www.ncbi.nlm.nih.gov). Actinomycetes have characteristic biological aspects such as mycelial forms of growth that accumulates in sporulation and the ability to form a wide variety of secondary metabolites including most of the antibiotics.
One of the major groups in actinomycetes is Streptomyces. Streptomyces contains 69-78 mol% of G+C. Substrate and aerial mycelium is highly branched. Substrate hyphae are 0.5-1.0 µm in diameter. In the colony ages aerial mycelia develop into chain of spores (conidia) by the formation of crosswalls in the multinucleated aerial filaments. Conidial wall are convoluted projection which together with the shape and the arrangement of the spore-bearing structure are characteristic of each species of Streptomyces (Anderson et al., 2001). It produces several antibiotics including of aminoglycosides, anthracyclins, glycopeptides, b-lactams, macrolides, nucleosides, peptides, polyenes, polyethers and tetracyclines (Sahin and Ugur, 2003).
Thus investigators turn towards Streptomyces and also other genera of actinomycetes such as Nocardia, Micromonospora, Thermoactinomycetes etc. for isolation of novel antibiotics. No doubt soil is the natural habitat of most of the microorganisms where vast array of bacteria, actinomycetes, fungi and other organisms exist and provided with suitable growth condition and ability to proliferate. Thus most actinomycetes contributing to antibiotic production are screened from soil (Williams and Khan, 1974).
Our prime focus is to find out the novel antibiotic with broad-spectrum antimicrobial activity from Streptomyces isolates of high altitude. And I will do further study in Streptomyces isolates of Khumbu region.
In, RLABB, The first work on the diversity of actinomycestes was started by Singh, D. and Agrawal, V.P. (2002). The research on actinomycetes form Mount Everest was then continued by Pandey, B., Ghimire, P. and Agrawal, V.P. (2004). Still the work is conducting by Baniya R, Guragain M, Sherpa C and Gurung T. Baniya found many actinomycetes with broad-spectrum antimicrobial activity. Among them most of actinomycetes are Streptomyces. Although more research have been done on antibiosis, classification of antibiotic groups and genetic characterization on the basis of 16s rRNA gene have to be done for significant use in medical field. 16s rRNA gene are highly conserved in bacteria. But contain three sequence variable regions among (α, β and γ shown in fig. 1). Among that variation in the sequence of α-(from nucleotide 982 to 998), β-(1102-1122) regions are used of differentiate genus and γ-(158-203) region for the detection of species of bacteria (Anderson et al., 2001). Hence the study will explore more about the genetic property of Streptomyces and chemical property of antibiotics produced by them.

Fig: Secondary structure of 16S rRNA from Streptomyces coelicolor.
Objectives
1. Biochemical and Genetic Characterization of Streptomyces
2. Chemical Characterization of antibiotics from the Streptomyces isolates
Methodology
1. Isolation Purification of Streptomyces
Soil samples will be obtained from Research Laboratory for Agricultural Biotechnology and Biochemistry (RLABB). Isolation of actinomycetes will be performed by soil dilution plate technique using Starch-Casein Agar (Singh and Agrawal, 2002 & 2003). Actinomycetes on the plates will be identified as colored, dried, rough, with irregular/regular margin; generally convex colony as described by Williams and Cross (1971). Streak plate method will be used to purify cultures of actinomycetes (Williams and Cross, 1971, Singh and Agrawal 2002; Agrawal 2003). After isolation of the pure colonies based on their colonial morphology, colour of hyphae, color of aerial mycelium, they will be individually plated on another but the same agar medium.
2. Morphological and Biochemical characterization:
3. Screening of Streptomyces for antimicrobial activity:
3.1 Primary screening
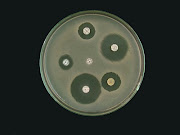
Primary screening of pure isolates will be determined by perpendicular streak method on Muller Hinton agar (MHA). In vitro screening of isolates for antagonism: MHA plates will be prepared and inoculated with Streptomyces isolate by a single streak of inoculum in the center of the petridish. After 4 days of incubation at 28 °C the plates were seeded with test organisms (Bacillus subtilis, Staphylococcus aureus, Enterobacter aerogens, Escherichia coli, Klebsiella species, Proteus species, Pseudomonas species, Salmonella typhi and Shigella species) by a single streak at a 90° angle to Streptomyces strains. The microbial interactions were analyzed by the determination of the size of the inhibition zone.
3.2 Secondary screening
Secondary screening is performed by agar well method against the standard test organism. Fresh and pure culture of each strain from the primary screening will be inoculated in starch casein broth and incubated at accordingly for 7 days in water bath shaker. Growth of the organism in the flask will be confirmed by the visible pellets, clumps or aggregates and turbidity in the broth. Contents of flasks will be filtered through Whatman no.1 filter paper. The filtrate will be used for the determination of antimicrobial activity against the standard test organisms.
4. Genetic Characterization
4.1 DNA extraction: Individual strains will be mass cultured in SC-broth by incubation the broth in shaker water bath for 5-6 days at 28C. Total DNA from corresponding strains will be extracted as described by a modified version of procedure of Kutchma et al. (1998) (Appendix-II).4.2 DNA polymorphisms: DNA polymorphisms among the strains will be studied by Specific-PCR using Universal primers 8F and 1491R that will amplify specifically 16S rDNA. The amplified band will be sequenced for sub species identification (Rivas et al., 2001) (Appendix-III)
5. Fermentation process
Isolates showing the broad-spectrum antimicrobial activity are grown in submerged culture in 250 ml flasks containing 50 ml of broth describe in Sahin & Ugur,2003. The flasks are inoculated with 1ml of active Streptomyces culture and incubated at 28ºc for 7 days with shaking at 500 rpm. After fermentation, fermented broth will filtered through Whatman no.1 filter paper.
6. Extraction of antimicrobial metabolites
Antibacterial compound will be recovered from the filtrate by treating twice with one volume of ethyl acetate (Busti et al., 2006). And after evaporation residue will be used for determination of antimicrobial activity, minimum inhibitory concentration and to perform bioassay of antibiotic (Pandey et al., 2004).
7. Thin Layer Chromatography and Bioautography
Silica gel plates, 10 X 20 cm, 1mm thick, are prepared. They are activated at 150°C for half an hour. Ten micro-liters of the ethyl acetate fractions and reference antibiotics are applied on the plates and the chromatogram is developed using chloroform: methanol (4:1) as solvent system. The plates are run in duplicate; one set is used as the reference chromatogram and the other is used for Bioassay of antibiotic. The spots in the chromatogram are visualized in the iodine vapour chamber and UV chamber (Thangadural et al., 2002 and Pandey et al., 2004).
Expected Outcomes
Being majority of antibiotics producing Actinomycetes are Streptomyces in this research work we have selected two Streptomyces sps with broad spectrum antimicrobial activity. Since these species are from very cold Everest region the organisms as well as antibiotics produced by them may be novel ones.
References
Anderson AS and Wellington EMH (2001) The taxonomy of Streptomyces and related genera. Int J Syst Evol Microbiol 51:797–814
Bergey's manual of determinative bacteriology 2000 Actinomycetales, 9th edition.
Holt, J.G. 1989 Bergey's manual of systematic bacteriology, vol 4, ed. S.T. Williams and M.E. Sharpe, Baltimore, Md: Williams and Williams.
Kuster, H.J. 1968 Uber die Bildung Von Huminstoffen durch Streptomyceten. Landwirtsch. Forsch, 21:48- 61
Kutchma, A.J., Roberts, M.A., Knaebel, D.B. and Crawford, D.L. (1998) Small scale isolation of genomic DNA from Streptomyces mycelia or spores. Biotechniques, 24:452-457.
Nisbet, L.J. and F.M. Fox 1991 The importance of microbial biodiversity to biotechnology, In, The biodiversity ofmicroorganisms and invertebrates : its role in sustainable Agriculture, ed.D.L. Hawksworth, 229-224, CAB International.
Pandey, B., Ghimire, P. and Agrawal, V.P. (2004) Studies on Antibacterial Activity of Soil from Khumbu Region of Mount Everest, a paper presented in International Conference on The Great Himalayas : Climate, Health, Ecology, Management and Conservation, Kathmandu, January 12 -15, 2004
Rivas R., Velázquez E., Valverde A., Mateos P.F. and Molina E. M. 2001 A two primers random amplified polymorphic DNA procedure to obtain polymerase chain reaction fingerprints of bacterial species Electrophoresis 22, 1086–1089
Singh, D. and Agrawal, V.P. (2002) Microbial Biodiversity of Mount Everest Region, a paper presented in International Seminar on Mountains - Kathmandu, March 6 – 8, 2002 (organized by Royal Nepal Academy of Science and Technology )
Singh, D. and Agrawal, V.P. (2003) Diversity of Actinomycetes of Lobuche in Mount Everest Proceedings of International Seminar on Mountains – Kathmandu, March 6 – 8, 2002 pp. 357 – 360.
Williams, S.T. and T. Cross 1971 Actinomycetes. In: J.R. Norris, D. W. Robbins, (eds), Methods in microbiology, vol.4. London, 295-334, Academic Perss, NewYork.
www.ncbi.nlm.nih.gov.
Saturday, July 14, 2007
A DEMONSTRATIVE MANUAL FOR BASIC MOLECULAR BIOLOGY PRACTICAL
BASIC MOLECULAR BIOLOGY PRACTICAL
FOR THE STUDENTS OF
DEPARTMENT OF MEDICAL MICROBIOLOGY
NOBEL COLLEGE
POKHARA UNIVERSITY
Jul 1-6, 2007
Prepared by
Kiran Babu Tiwari
Asst. Prof. of Microbiology, USC
Research Scientist, RLABB
In association with
Upendra Thapa Shrestha
Nirajan Bhattarai
Vijayendra Agrawal
(Research Scientists, RLABB)
Reference:
Professor Dr. Vishwanath Prasad Agrawal
Executive Director
Research Laboratory for Biotechnology and Biochemistry (RLABB)
www.uscollege.edu.np/rlabb
http://www.rlabb.com.np/
http://www.vpagrawal.com/
Acknowledgement
We are indebted to
Prof. Dr. Vishwanath P. Agrawal,
Executive Director, Research Laboratory for Biotechnology and Biochemistry (RLABB);
Director, Universal Science College; and
Academician, National Academy of Science and Technology (NAST)
for providing the facilities to conduct the experiments enlisted in this manual.
CONTENTS
TITLE PAGE
ACKNOWLEDGEMENT
CONTENTS
JULY 1:
JULY 2:
EXPT 2. ISOLATION AND PURIFICATION OF RNA
JULY 3:
EXPT 5. RESTRICTION DIGESTION OF DNA
JULY 5:
JULY 6:
1. Principle of nucleic acids isolation from bacteria
Nucleic acids are present as nucleoprotein complexes in cells. The major problems encountered in isolation of pure and intact DNA or RNA molecules are: degradation of high molecular weight nucleic acids by mechanical damage or by hydrolytic action of nucleases, contamination of DNA preparations with RNA and vice versa and contamination of nucleic acids with proteins, polysaccharides and other high molecular weight compounds. Methods have been devised for isolation of nucleic acids from different sources taking adequate precautions to eliminate or minimize the above problems.
The main steps involved in isolation of nucleic acids are:
(a) Disruption of cells: Disintegration of bacterial cells can be achieved by treating them with cell coat hydrolyzing enzyme, i.e. lysozyme in presence of a detergent. at low temperature in buffers containing EDTA (chelates Mg2+ ions which are required for DNase activity). For isolation of RNA, and inhibitor of RNase such as bentonite, diethylpyrocarbonate, placental RNase inhibitor etc. is included in the extraction buffer.
(b) Dissociation of nucleo-protein complexes: The approach employed is such that the proteins either get dissociated or degraded while nucleic acids remain unaffected and intact. This is generally achieved by using detergents like SDS, phenol or broad-spectrum proteolytic enzymes such as pronase or proteinase K. Alkaline pH and high concentration of salts improve efficiency of the process.
(c) Removal of contaminating materials and precipitation of nucleic acids: Proteins are removed by treatment with phenol or mixture of chloroform-isoamyl alcohol or phenol-chloroform. Upon centrifugation, the denatured proteins form a layer at the interface between upper aqueous and lower organic phases, lipids and other contaminants remain in the same organic phase while nucleic acids are recovered in the aqueous phase from which they are precipitated with ethanol. For isolation of DNA, the contaminating RNA is removed by selective salt precipitation or treatment with DNase-free RNase. Conversely while isolating RNA, the preparation is incubated with DNase for eliminating DNA as an impurity.
(d) General precautions while handling nucleic acids:
1. All glasswares and solutions (except organic solvents) should be sterilized.
2. Gloves should be worn to avoid contamination of the experimental material and apparatus with nucleases with occur in fair abundance in skin exudates.
3. Phenol causes severe burns and phenol-containing solutions should, therefore, be handled with care. Thoroughly rinse the burns with large volume of water. Do not use ethanol.
4. For work with RNA, rinse all glasswares with 1% diethylpyrocarbonate solution to inactivate RNase and then autoclave them.
2. Basic components for Molecular Biology experiments
LB (Lauria-Bertani) broth: 0.5% NaCl, 1% Tryptone, 0.1% Yeast extract
Overnight Log- phase culture
Solution I (Lysis buffer): 25mM Tris, 50mM Glucose, Lysozyme [10mg/ml stock; GNB (Gram Negative Bacteria) 0.5mg/ml, GPB (Gram Positive Bacteria) 3-5mg/ml], pH 8.0
Solution II (Lysis): (a) for plasmid isolation: 0.2M NaOH, 10% SDS (b) for genomic DNA isolation: 10% SDS only.
Proteinase K: 10mg/ml stock, 10-20mg/65°C/3hrs
Solution III: 3M Sodium acetate, pH 4.8 by acetic acid
Phenol: distilled, protein precipitant
Chloroform: protein precipitant, phenol solvent
RNase: 5mg/ml stock, 1-2ml
Absolute ethanol/Isopropanol: DNA precipitant, 2.5vol of ethanol or 1vol of isopropanol
70% ethanol: washing DNA precipitant
TE buffer: DNA resuspending buffer, 10mM Tris, 1mM EDTA, pH 8.0; Autoclave before use
TAE buffer: Electrophoresis, 50X, Tris, 24.2gm; acetic acid, 5.71ml; EDTA (0.5M), 11.1ml; DW, 100ml; pH 8.0; Autoclave before use
Loading dye: 6X; 50% Glycerol, 6.0ml; 2%BPB (Bromophenol Blue), 1.0ml; DW, 3ml; Always use sterile DW
Ethidiun Bromide (EtBr)*: 10mg/ml stock (Final concentration: 0.5mg/ml)
λ/HindIII Marker: 23.13Kb, 9.42Kb, 6.56Kb, 2.32Kb, 2.07Kb, 0.56Kb and 0.13Kb
Restriction enzymes: EcoRI and HindIII (10U/ml each)
Restriction enzyme buffers for EcoRI and HindIII (10X each)
Sterile Double distilled water (DDW)
Water bath
Cold centrifuge (upto 20000rpm)
Micropipettes and sterile tips
*Note: EtBr is carcinogen, so, handle with gloves
Day 2
Expt. 1. Genomic DNA extraction from bacteria
-Take 1.5ml of overnight LB-broth culture of bacteria in a MFT (Microfuge tube) and spin at 5000rpm for 10min.
-Remove the supernatant and spin once with same volume as above to collect more cell mass.
-Add 100µl Sol. I and keep for 30min at RT (Room temperature).
-Add 1/10 vol. of 10% SDS and swirl to mix.
-Add Proteinase K (1-2 µl) and incubate for 30min at 37ºC with gentle shaking.
-Add 1-2µl of RNase and incubate for 10min at RT.
-Add equal vol. of Phenol:Chloroform (1:1), mix gently and keep for 10min at RT.
-Centrifuge at 8000rpm for 10min at 4ºC and collect the supernatant in a new sterile MFT.
-Add equal vol. of 3M sodium acetate, mix and stand it for an hour in cold.
-Spin at 10000rpm at 4ºC for 15min and wash the pellet with 70% ethanol.
-Dissolve the pellet collected during spin in 50µl TE and store in deep freeze.
Expt. 2. RNA extraction from bacteria
-Take 1.5ml of overnight LB-broth culture of bacteria in a MFT and spin at 5000rpm for 10min.
-Remove the supernatant and spin once with same volume as above to collect more cell mass.
-Remove the supernatant.
-Add 100µl Sol. I and keep for 30min at RT.
-Add 1/10 vol. of 10% SDS and swirl to mix.
-Add Proteinase K (2 µl) and incubate for 30min at 37ºC with gentle shaking.
-Add equal vol. of Phenol:Chloroform (3:1) solution and mix gently.
-Centrifuge at 8000rpm for 10min at 4ºC and collect the supernatant in a new sterile MFT.
-Add equal vol. of 3M sodium acetate and/or two vol. of absolute ethanol. Mix and stand it for 1-2 hour in cold.
-Spin at 15000rpm at 4ºC for 15min and wash the pellet with 70% ethanol.
-Dissolve the pellet collected during spin in 50µl TE (pH 7.0) and store in deep freeze.
Day 3
Expt 3. Plasmid DNA extraction from bacteria
-Take 1.5ml of overnight LB-broth culture of bacteria in a MFT and spin at 5000rpm for 10min.
-Remove the supernatant and spin once with same volume as above to collect more cell mass.
-Remove the supernatant.
-Add 100µl Sol. I and keep for 30min at RT.
-Vortex for a while and add Proteinase K (2µl) and incubate for 30min at 37ºC with gentle shaking.
-Add freshly prepared Sol. IIa (200µl) and mix gently (Do not vortex).
-Add ice cold Sol. III (150µl) and mix gently (Do not vortex).
-Add 1-2µl of RNase and incubate for 10min at RT.
-Add equal vol. of Phenol: Chloroform (1:1), mix gently and keep for 10min at RT.
-Centrifuge at 8000rpm for 10min at 4ºC and collect the supernatant in a new sterile MFT.
-Add equal vol. of isopropanol and stand it for an hour in cold.
-Spin at 13000rpm at 4ºC for 15min and wash the pellet with 70% ethanol.
-Dissolve the pellet collected during spin in 50µl TE and store in deep freeze.
Day 4
1. Remove the DNA preparation from the freeze and thaw it.
2. Transfer 495μl TE buffer to a quartz cuvette and add 5μl of the DNA preparation. Mix well.
3. Set the spectrophotometer to 260nm and blank the instrument with TE buffer.
4. Measure the absorbance (A260) of the DNA dilution.
5. Repeat steps 3 & 4 with the spectrophotometer set at 280 nm.
6. Calculate the DNA concentration from A260. [µg/ml = A260 X dilution X 50].
7. Calculate A260/A280 in order to estimate the purity of the DNA preparation (Pure DNA has a A260/A280 ratio of 1.8 – 2.0).
1. Dilute the DNA extracts, Marker/s and enzymes in suitable solvents accordingly.
2. Calculate the volume for digestion reaction as shown in the table given below.
Components............ Rxn. .........1 Rxn. 2
λ DNA (1µg/µL) ............10 µL ..............-
DNA extract (1µg/µL) ...- ....................10 µL
HindIII (10U/µL) .........1 µL ................1 µL
HindIII buffer (10X) ....1 µL ................1 µL
DW ..................................8 µL ................8 µL
Total ...............................20 µL ..............20 µL
3. Perform digestion reaction by mixing the components in sterile MFT.
4. Briefly centrifuge the contents and keep at 37ºC for 6hrs.
5. Perform agarose gel electrophoresis to interpret the results.
Day 5
Expt. 6. Agarose electrophoresis of DNA
1. Prepare 0.8% agarose gel in 1X TAE buffer (28ml).
2. Dissolve agarose completely in micro-oven and cool to 600C.
3. CAREFULLY, add EtBr into the gel solution to final concentration of 0.5µg/ml.
4. Position a comb in the mold. Pour into gel mold and let it cool for 30 minutes.
5. Pour the TAE buffer into the gel buffer reservoir.
6. Prepare sample taking 20µl of DNA sample and mix with 4µl blue juice.
7. Carefully remove the comb.
8. Load the DNA (15 µl) in the wells, flanking wells with similarly processed DNA size standard.
Note: the amount of the sample that can be loaded in a well depends on the thickness of the gel as well as dimensions and placing of the comb.
9. Put the lid on the gel apparatus; attach the electrodes and adjust voltage to 100 volts.
10. Allow the gel to run until line of blue juice is visible near the end of the gel.
11. Turn off the current and visualize the gel in UV transilluminator.
12. Interpret the results.
Day 6
Expt 7. RAPD-PC(Randomly Amplified Polymorphic DNA – Polymerase Chain Reaction)
1. Thaw the DNA extract and dilute in sterile TE to final concentration with10ng/µl.
2. Prepare reaction mixture as given below:
Components---------- Volume (µl)
PCR buffer (pH 8.3) ----------5
dNTP (2.5mM each) ---------4
Taq polymerase (1U/µl) -----1
Template DNA (10ng/µl) ----1
RAPD-Primer (10µM) -------8
10% DMSO ------------------5
DDW ------------------------26
Final Volume-----------------50
3. Operate the thermal cycler program as given below:
Initial denaturation------- 94ºC-------5mi -----Single step
Denaturation -------------94ºC -------1min
Annealing ----------------36ºC -------1min ----30 Cycles
Extension ----------------72ºC -------2min
Final extension -----------72ºC -------5min ----Single step
4. Perform agarose gel electrophoresis as described in Expt. 6.
Sunday, July 8, 2007
OPTIMIZATION OF RAPD-PCR FOR GENETIC FINGERPRINTING OF BACILLUS THURINGIENSIS
(1) Research Laboratory for Agricultural Biotechnology and Biochemistry (RLABB), Universal Science College, Maitidevi, Kathmandu, Nepal;
(2) Central Department of Microbiology, Tribhuvan University, Kirtipur, Nepal
* Corresponding author: gyan633413@gmail.com
ABSTRACT
Tuesday, June 19, 2007
Delta-endotoxin Immuno Cross-reactivity of Bacillus thuringiensis Isolates Collected from Khumbu Base camp of Mount Everest Region
Strong mosquitocidal Bacillus thuringiensis from Mt. Everest
(1) Research Laboratory for Agricultural Biotechnology and Biochemistry (RLABB), Universal Science College, Maitidevi, Kathmandu, Nepal;
(2) Central Department of Microbiology, Tribhuvan University, Kirtipur, Nepal
*Address for Correspondence: Prof. Dr. Vishwanath Prasad Agrawal, RLABB, Tel: +977-1-4442775, E-mail: vpa@wlink.com.np
Ben-Dov E, Wang Q, Zaritsky A, Manasherob R, Barak Z, Schneider B, Khamraev A, Baizhanov M, Glupov V, Margalith Y. Multiplex PCR screening to detect cry9 genes in Bacillus thuringiensis strains. Appl Environ Microbiol 1999; 65: 3714-6.
Bergey’s Manual of Systematic Bacteriology, Volume 2, 1986.
Dulmage HT. Production of spore-delta-endotoxin complex by variants of Bacillus thuringiensis in two fermentation media. J Invertebr Pathol 1970; 16: 385-9.
Neppl CC (2000). Managing Resistance to Bacillus thuringiensis Toxins. Environmental Studies University of Chicago.
Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler DR and Dean DH. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev 1998; 62: 775-806.
Shrestha UT, Sahukhal GS, Pokhrel S, Tiwari KB, Singh A and Agrawal VP. Delta- endotoxin immuno cross-reactivity of Bacillus thuringiensis isolates collected from Khumbu base camp of Mount Everest region. J Food Sci Technol Nepal 2006; 2: 128-131.
Travers RS, Martin PA and Reichelderfer CF. Selective Process for Efficient Isolation of Soil Bacillus spp. Appl Environ Microbiol 1987; 53: 1263-6.
Sunday, June 17, 2007
Cloning of cry3 fragment in Escherichia coli
(1) Department of Biochemistry, Universal Science College, Pokhara University, Kthmandu, Nepal;
(2) Research Laboratory for Agricultural Biotechnology and Biochemistry (RLABB), Kathmandu, Nepal;
(3) Department of Enzyme Engineering, Seoul National University, Korea;4Department of Life Sciences, Ben-Gurion University of the Negev, Be’er-Sheva 84105, Israel*
Correspondence Address: Dr. Vishwanath P. Agrawal, Professor of Biochemistry, Research Laboratory for Agricultural Biotechnology and Biochemistry (RLABB), Kathmandu, Nepal.Email: vpa@wlink.com.np. Contact: +977-1-2110043
ABSTRACT
Bacillus thuringiensis was isolated and purified from the soil sample collected from Khumbu, Mt. Everest base camp. Total DNA was extracted and PCR was done using nine universal primers for cry1 to cry9. A specific band of about 300bp was amplified with universal primer 3. DNA library was created by digesting the DNA with HindIII, ligation into pUC18 followed by transformation of Escherichia coli HB101. Using universal primer 3 for PCR, 100 out of 1000 clones prepared were screened and three were found to posses cry3 specific fragment of the same size as before. The cry3 specific fragment was cloned, extracted, purified and sent for sequencing. As the bacterium was isolated from high altitude, the gene may be novel with promising for biological control and management of insects.
McGaughey WH and Whalen ME. Managing insect resistance to Bacillus thuringeinsis Toxins. Science 1992; 258: 1451-5.
Sambrook J, Fritsch EF and Maniatis T. Molecular cloning: A laboratory manual. 2nd Ed. Cold Spring Harbor Laboratory press, Cold Spring Harbor, New York. 1989.
Schnepf HE and Whiteley HR. Cloning and expression of Bacillus thuringeinsis crystal protein gene in E. coli. Biochemistry 1981; 78: 2893-7.
Shreshtha UT, Sahukhal GS, Pokhrel S, Tiwari KB, Singh A and Agrawal VP. Delta- endotoxin immuno cross-reactivity of Bacillus thuringiensis isolates collected from Khumbu base camp of Mount Everest region. J Food Sci Technol Nepal 2006; 2: 128-131.
Thermostable glucose isomerase from psychrotolerant Streptomyces species
(1) Universal science College, Pokhara University, Kathmandu, Nepal
(2) Research Laboratory for Agricultural Biotechnology and Biochemistry, Kathmandu, Nepal
(3) Department of Enzyme Engineering, Seoul National University, Korea
Corresponding Address: Dr. Vishwanath P. Agrawal, Professor of Biochemistry, Universal Science College, Pokhara University, Kathmandu. Email: vpa@wlink.com.np
ABSTRACT
Glucose isomerase (EC 5.3.1.5) was extracted from Streptomyces spp., isolated from Mt. Everest soil sample, and purified by ammonium sulfate fractionation and Sepharose-4B chromatography. A 7.1 fold increase in specific activity of the purified enzyme over crude was observed. Using glucose as substrate, the Michaelis constant (KM) and maximal velocity (Vmax) were found to be 0.45M and 0.18U/mg. respectively. The optimum substrate (glucose) concentration, optimum enzyme concentration, optimum pH, optimum temperature, and optimum reaction time were 0.6M, 62.14μg/100μl, 6.9, 70ºC, and 30 minutes, respectively. Optimum concentrations of Mg2+ and Co2+ were 5mM and 0.5mM, respectively. The enzyme was thermostable with half-life 30 minutes at 100ºC.
INTRODUCTION
Most commercially available GI has been isolated from mesophilic microorganisms, including Streptomyces, Actinoplanes, Flavobacterium and Bacillus spp.3 GIs are homotetramer with 45kDa or 49kDa, the former being more conservative.5- 9 Most of the GIs are not highly thermostable (limited to 60ºC only) and less active at neutral. Thermostable GIs with neutral or slightly acidic pH optima have a potential for industrial applications. The thermo-acid-stable GI allow for faster reaction rates, higher fructose concentration at equilibrium, higher process stability, decreased viscosity of substrate and product streams, and reduced by-product formation.10
Owing to the industrial significance of the enzyme, GI from various microorganisms has been studied and their catalytic and physicochemical properties have been reviewed.11,12 Thermophilic microorganisms produce industrially thermostable enzymes which have been evolved and adapted to the extreme environment of their natural habitats. As the Research Laboratory for Agricultural Biotechnology and Biochemistry (RLABB) has been studying and exploiting the actinomycetes’ population diversity from high altitude ecological niche, this work was done on D-glucose isomerase of the psychrotolerant Streptomyces spp. isolated from the soil sample collected from Khumbu, Mount Everest base camp.
METHODOLOGY
Culture: Streptomyces spp. Lob 15.4, isolated from soil samples collected from Lobuche, Mt. Everest base camp, was revived by inoculating spores into a 250 ml conical flask containing 50 ml of culture medium (1% tryptone, 0.7% yeast extract, 1% xylose and 0.1%MgSO4.7H2O, pH 7.0-7.2) followed by incubating at 28ºC in a shaker waterbath (200rpm) for 4 days.13
Enzyme preparation: Streptomyces cells were collected by centrifugation, washed several times with deionised water and homogenized in vertexer in 0.1M phosphate buffer (pH 7.0) containing 5mM MgSO4, 0.5mM CoCl2 and 1mM PMSF. Cells were disrupted in a bath sonicator for 30 min with ice and centrifug at 10000 rpm for 20 min at 4ºC to obtain enzyme supernant.13
Enzyme assay: A 100ml of the enzyme was incubated in 900ml phosphate buffer (pH 7.0) containing 5mM MgSO4, 0.5mM CoCl2 and 0.8M glucose at 37ºC for 40 minutes, followed by keeping the tubes in an ice bath. The amount of the product, fructose, was determined by Seliwanoff’s method.10
Protein determination: Protein content in the supernatant was determined by Bradford assay.14
Enzyme purification: Ammonium sulphate was added to the crude enzyme extract to 45% saturation, incubated for an hour at 4ºC with gentle mixing. The precipitate was collected by centrifugation at 10,000 rpm for 20 min at 4ºC and dissolved in 0.1M phosphate buffer (pH 7.0) containing 5mM MgSO4, 0.5mM CoCl2. The ammonium sulphate concentration was increased stepwise to 60%, 75% and finally to 90% saturation; and the precipitates were harvested accordingly. The fraction containing glucose isomerase activity was pooled and dialyzed overnight against 0.1M-phosphate buffer (pH 7.0).15 Then, a Sepharose 4B column (3.2 by 38.5cm) was prepared and equilibrated with 0.05M phosphates buffer containing 0.15M NaCl. The dialyzed enzyme was applied to the column and eluted with the phosphate buffer. Fraction containing glucose isomerase activity was collected, concentrated with ammonium sulphate and dialyzed against 0.1M phosphate buffer (pH 7.0).15 The purification steps were monitored by SDS-PAGE16 and Native-PAGE17.
Optimization: Optimization was done in phosphate buffer (pH 7.0) containing 5mM MgSO4, 0.5mM CoCl2 at 37ºC for 40 min. unless mentioned otherwise. Glucose (substrate) from 0.1-1.0M and enzyme from 6-124µg were mixed in the respective optimization reaction. Phosphate buffers from pH 4-10 were used to optimize pH of the respective reaction with 0.6M glucose. Time and temperature were optimized by incubation the respective reaction mixtures for 10 to 60 minutes and 30 to 90ºC in phosphate buffer with pH 6.9. To optimize Mg2+ and Co2+ concentrations, 0.05-10mM ions were mixed in the respective reaction buffer. Amount of product (fructose) produced was determined by Seliwanoff’s method.10
Half-life and Thermal stability were determined by measuring residual activity under optimum assay condition after pre-incubation of the enzyme- at 100ºC for 5-30 minutes for half life and at 40-90ºC for 30 to 150 minutes for thermal stability.15
RESULT
The production of glucose isomerase from Streptomyces species has been documented by several investigators.18,19 The sample sonicated for 20minutes showed maximum enzyme activity.(13)
Chou and Anderson also found glucose isomerase activity in 90% ammonium sulfate saturation.20 To get more purified form of the enzyme, the chromatography has to be done several times. Chen and Anderson reported that the enzyme was purified using DEAE –Sephadex A-50 and the purification was about 12.6 fold over the crude.20 The enzyme activity was stable at 37ºC; therefore, all steps of purification were performed at that temperature. The purified fraction had specific activity 0.490U/mg and crude enzyme had 0.069 U/mg, suggesting a 7.1 fold increase in specific activity of the purified enzyme over crude was observed.
Homogeneity of the purified enzyme was determined by Sodium dodecyl sulphate -polyacrylamide gel electrophoresis (SDS-PAGE). The purified enzyme was homogeneous by the detection of a single protein band on SDS-PAGE and Native-PAGE. Approximately, mol. wt. of 200kD protein band on Native-PAGE and about 50kD on SDS-PAGE was determined, suggesting that the enzyme to be tetramer.
The enzyme also called as Xylose isomerase (XI) as it converts xylose to xylulose besides converting glucose to fructose. Hence, xylose was used as the inducer of the enzyme in the culture medium. The enzyme was, then, optimized using glucose as a substrate. The optimum glucose concentration was 0.6M. The Michaelis constant (KM) and maximal velocity (Vmax) were found to be 0.45M and 0.18U/mg. respectively. In the other studies the KM value upto 0.2M10 to 0.167M21 was also been reported. Lama et al. reported Vmax of 6.3U/mg.21 The enzyme with lower KM and higher Vmax towards substrate is more preferred for exploitation of enzyme behavior, suggesting the enzyme extract in the work may not be as competent to that obtained from mesophilic or thermophilic bacteria which are more suitably adapted to higher temperature.
The optimum pH is the ranges between pH 7.0 to 9.0.4 The optimum pH of the glucose isomerase is slightly acidic, pH 6.9. It was apparently lower than that of enzymes from other Streptomyes species.22 Therefore, a low pH optimum is an attractive property for enzyme application because the use of the enzyme at neutral or low pH prevents the formation of by-product, psicose.
Most of glucose isomerase isolated to date showed an optimum temperature around 80ºC.13 Most of the industrially exploitation of the enzyme is done at 60ºC, as Hodge indicated that degradation of ketoses occurs at high temperatures, characterized by pronounced discoloration of an aqueous sugar solution. Interestingly, in this study, the optimum temperature of the enzyme from the cold tolerant bacteria was 70ºC. This may be due to conservation of the gene in bacterial population.6-9 Lama et al. also documented that the kinetic characteristics for XI or GI were similar to XI from distantly related bacteria.21 The optimum temperature of the GI explored that psychrotolerant organisms may have thermostable proteins.
The optimum reaction time of the enzyme was 30min, similar to most of the enzymes from diversed bacteria.1,4,20 Compared to the half life reported by Chou et al13, 120h at 70ºC, the half life of the GI in this work was 30 minute at 100ºC, suggesting to be a quite thermostable one.
Glucose isomerases typically require the presence of divalent metal cations such Mg2+ or Co2+ as essential cofactors for their catalytic activity.23 Treatment of purified enzyme with EDTA resulted in an almost complete loss of enzyme activity. However, the activity could be restored by the addition of metal ions. In particular, increasing amounts of Mg2+ or Co2+ (each up to 10mM) were able to restore only 60-80% of the original xylose isomerase activity. Lama et al. reported, for glucose isomerase, 10mM Mg2+ was required to restore 80% of the original activity.21 As is common with other isomerases, 10mM Mg2+ plus 1mM Co2+ restored total glucose isomerase activity. However, the lower values of the cations observed in this study, suggested that the enzyme might be adapted in the ecological niche.
ACKNOWLEGMENT:
We express our especial thank to Mr. Yogan Khatri, Mr. Deepak Singh and Rajendra Aryal for collecting soil samples from Mount Everest region and all the staffs of RLABB.
REFERENCES:
1. Chen WP (1980). Glucose Isomerase. Proc Biochem 15: 30-41.
3. Bhosale SH., MB Rao and VV Deshpande (1996). Molecular and industrial aspects of glucose isomerase. Microbiol Rev 60:280-300.
4. Lee C and JG Zeikus (1991). Purification and characterization of thermostable glucose isomerase from Clostridium thermosulfurogenes and Thermoanaerobacter stain B6A. Biochem J 274: 565-571.
5. Kwon, HJ., Kitada, M., Horikoshi K. (1987).Purification and properties of D-xylose isomerase from alkaliphilic Bacillus no KX-6. Agric Biol Chem 51:1983-1989.
6. CarrelHL., BH Rubin, TJ Hurley and JP Glusker (1984). X-ray crystal structure of D-xylose isomerase at 4-A resolution. J Biol Chem 259:3230-3236.
7. Farber GK, A Glasfeld, G Tiraby, G Ringe and GA Petsko (1989). Crystallographic studies on the mechanism of xylose isomerase. Biochemistry 28: 7289-7297.
8. Dauter Z, M Dauter, J Hemker, H Witzel and KS Wilson (1989) Crystallization and preliminary analysis of glucose isomerase from Streptomyces albus. FEBS Lett 247:1-8.
Wednesday, June 13, 2007
Hi all,
Bacteria in Photos