Corresponding Author: Prof. Dr. Vishwanath Prasad Agrawal, Executive Director, Research Laboratory for Biotechnology and Biochemistry (RLABB), Maitidevi, Kathmandu, email: vpa@wlink.com.np
ABSTRACT
Streptomyces spp. (Lob18.2b), isolated from soil sample from Everest Base Camp, was taken from Research Laboratory for Biotechnology and Biochemistry (RLABB). The isolate was found to inhibit Salmonella paratyphi, Salmonella typhi, Proteus mirabilis, Proteus vulgaris, Shigella sonnei, Klebsiella pneumoniae, Klebsiella oxytoca, Escherichia coli, Bacillus subtilis and Staphylococcus aureus on primary screening. Secondary screening was done using fermented starch casein broth of the streptomycete to its stationary phase culture. The antibacterial was highly effective against all susceptible Gram negative bacteria except Proteus spp. Gram positive bacteria were relatively lesser sensitive. Pseudomonas aeruginosa was resistant. Aqueous fraction of the antibacterial was effective than that of organic fraction. Thin layer chromatography revealed that the test compound was relatively nonpolar compared to the known antibiotics. Among the tested standard antibiotics, the chemical characteristic of the antibacterial agent was comparable to streptomycin.
Keywords: Antibacterial agent, fermentation, secondary screening, Streptomyces spp., Thin Layer Chromatography
INTRODUCTION
Actinomycetes comprise an extensive and diverse group of Gram-positive, aerobic, mycelial prokaryotes with high G+C content (>55%). The majority of actinomycetes are free living, saprophytic bacteria found widely distributed in soil, water and colonizing plants (Holt, 1989). Streptomyces species (GC%, 69-78) are the major group among actinomycetes (Holt, 1989, Korn-Wendisch and Kutzner, 1992). The genus Streptomyces was proposed by Waksman and Henrici (1943) and classified in the family Streptomycetaceae on the basis of morphology and subsequently cell wall chemotype. Streptomycetes are the major source (70%) of several commercially available antibiotics including aminoglycosides, anthracyclins, glycopeptides, β-lactams, macrolides, nucleosides, peptides, polyenes, polyethers and tetracyclines (Sahin and Ugur, 2003, Okami and Hotta, 1988; Baltz, 1998). The number of antimicrobial compounds reported from the species of this genus per year has increased almost exponentially for about two decades. Hence, these soil actinomycetes are preferentially screened for antibiotic production which has immense biotechnological value.
Various studies on cold tolerant actinomycetes are being conducted in Research Laboratory for Biotechnology and Biochemistry (RLABB) since 1999. Singh and Agrawal (2002 and 2003) had isolated and identified various actinomycetes from Khumbu, Everest Base Camp region. Pandey et al. (2004) did primary screening of some of the isolate for antibacterial activities. Hence, this work was designed with the objective to classify the antibiotic extracted from extreme environment (cold tolerant) inhabitant Streptomyces spp.
METHODOLOGY
Streptomyces spp. (Lob18.2b), isolated from soil sample from Everest Base Camp, was taken from Research Laboratory for Biotechnology and Biochemistry (RLABB). The isolate (primary screening) and its fermented secondary product (secondary screening) were assayed for antibacterial activity.
Primary screening: Primary screening of pure isolates was done by perpendicular streak method (Williams and Cross, 1971). Streptomycete was streaked on the nutrient agar as a straight line and incubated at 27ºC. After seven days of incubation, test organisms (Salmonella paratyphi, Salmonella typhi, Proteus mirabilis, Proteus vulgaris, Shigella sonnei, Klebsiella pneumoniae, Klebsiella oxytoca, Escherichia coli, Pseudomonas species, Bacillus subtilis and Staphylococcus aureus) were streaked perpendicular to the streak line. After 24 hours of incubation at 37ºC, the zones of inhibition (in mm) of the standard test organisms were measured.
Secondary screening: Secondary screening was performed by agar well method against the standard test organisms (Williams and Cross, 1971). Stationary phase culture of the streptomycete was prepared by inoculating the pure bacteria in Starch-Casein broth and incubating at 27ºC for two weeks in shaker water bath at 500 rpm. Supernatant was obtained by aseptic centrifugation (10,000 rpm for 10 minutes), a part of which was used for secondary screening by well cut method. The test organisms were grown in sterile nutrient broth at 37ºC for four hours to 0.5 McFarland Standard and swabbed onto Muller Hinton Agar surface. Agar wells were prepared using cork borer (diameter, 4mm). Subsequently, 100µl of the fermented broth was dispensed in the well and incubated at 37ºC for overnight and the zone of inhibition (in mm) were measured using a ruler.
Extraction of antimicrobial metabolites: Rest of the supernatant was mixed well with double volume of ethylacetate in a separating funnel and allowed to separate the two phages after one hour. Subsequently both upper (organic) and lower (aqueous) fractions were collected (Busti et al., 2006) and assayed for antimicrobial activity as above. The ethylacetate was evaporated at 40ºC and the residue was dissolved in sterile distilled water for assay.
Thin Layer Chromatography (TLC): Optimization of mobile phase (Butanol: acetic acid: water in two ratios of 4:1:5 and 2:1:8) for known antibiotics and test antibiotic was done by using 7.6 X 2.4 cm Silica gel plates, prepared and activated at 110°C for half an hour. Chromatogram was developed by loading 10µl of each fraction and running for half an hour. Spots on the plates were visualized in a iodine vapour chamber (Busti et al., 2006; Thangadural et al., 2002).
RESULTS
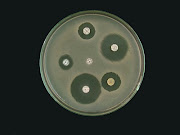
Primary Screening: The streptomycete inhibited all test organisms except Pseudomonas aeruginosa (Figure 1)
Secondary screening: All test organisms except Pseudomonas aeruginosa were inhibited by the fermented broth (Table 1). The aqueous fraction of the broth was better effective than organic one. Gram negative bacteria (GNB) were more susceptible compared to Gram positive (GPB) ones. Among the susceptible GNB, Proteus spp. were relatively lesser susceptible (Figure 2).
Table 1: Zone of inhibition of the fermented broth in secondary screening
TLC chromatogram: Single band was observed for all known and test antibiotics (Table 2, Figure 3).
Table 2: Rf –value of known and test antibiotics on TLC chromatogram
DISCUSSION
The isolate taken from RLABB was revived and subcultured to get pure and log phase growth for macroscopic, microscopic and biochemical assays in order to redefine its genera (Singh and Agrawal, 2002 and 2003) based on Bergey’s manual of systematic Bacteriology (Holt, 1989). During primary and secondary screening process, the test antibiotic was highly effective against the enteric GNB (Fig 1, Table 1). Enterobacteria are one of the major burden pathogens in clinical practices and hence, such a compound can be important discovery as it was extracted from high altitude streptomycete which may be novel antibiotic. The antimicrobial capacity of the compound looks similar to streptomycin (Greenwood, 1997, Brooks et al., 2001). Aminoglycoside is predominantly active against Gram negative enterobacteria and mycobacteria (Greenwood, 1997, Brooks et al., 2001). Aminoglycosides when combined with penicillins is effective against bacteraemia or endocarditis due to fecal streptococci and some GNB (Brooks et al., 2001). The chemical characteristic of the proposed streptomycin was further analyzed by TLC chromatogram findings. The test compound was chromatographed along with various classes of antibiotics in two solvent systems having different polarities (Table 2). The test compound was relatively nonpolar compared to the known antibiotics. The Rf values in the TLC chromatograph further characterize that the compound must come under aminoglycoside group, very much related to streptomycin. The respective differences in Rf values between streptomycin and the test compound indicate that the isolated antibiotic may have slight differences in its functional group(s) in molecular structure. Hence, this antibiotic should further be characterized in order to know its chemical features and clinical applications.
ACKNOWLEDGEMENT
The authors express full gratitude to CNR (Italy’s National Research Council) for supporting this work; and to Dr. Deepak Singh, Dr. Yogan Khatri and Dr. Rajindra Aryal for soil samples collection from Mount Everest region followed by isolation and identification of the Streptomyces spp. (Lob18.2b).
REFERENCES
Baltz RH (1998) Genetic manipulation of antibiotic producing Streptomyces. Trends in Microbiol 6: 76-83.
Busti E, Monciardini P, Cavaletti L, Bamonte R, Lazzarini A and Sosio et al. (2006) Antibiotic-producing ability by representatives of a newly discovered lineage of actinomycetes. Microbiology 152: 675-683.
Brooks GF, Butel JS and Morse SA (2001) Antimicrobial Chemotherapy. In: Jawetz, Melnick and Adelberg Medical microbiology. 22nd Ed. International Edition. Lange Medical Books / McGraw Hill Publication.
Greenwood D (1997) Antimicrobial agents. In: Greenwood D, Slac RCB and Peutherer JF (Eds.) Medical Microbiology, 15th Ed, ELST with Churchill Livingstone. Pp. 50.
Holt JG (1989) Bergey's manual of systematic bacteriology, vol 4, Ed. S.T. Williams and M.E. Sharpe, Baltimore, Md: Williams and Williams.
Korn-Wendisch F and Kutzner HJ (1992) The family Streptomycetaceae. In The Prokaryotes, pp. 921-995. Edited by A. Balows, H. G. Trus per, M. Dworkin, W. Harder & K. H. Schleifer. New York: Springer.
Okami Y and K Hotta (1988) Search and discovery of new antibiotics. p. 33-67. In M. Goodfellow, S.T. Williams, and M. Mordarski (eds.), Actinomycetes in Biotechnology.
Pandey B, Ghimire P and Agrawal VP (2004) Studies on Antibacterial Activity of Soil from Khumbu Region of Mount Everest, a paper presented in International Conference on The Great Himalayas Climate, Health, Ecology, Management and Conservation, Kathmandu, January 2-15.
Sahin N and Ugur A (2003) Investigation of the Antimicribial Activity of some Streptomyces isolates. Turk J Biol 27: 79-84.
Singh D and Agrawal VP (2002) Microbial Biodiversity of Mount Everest Region, a paper presented in International Seminar on Mountains - Kathmandu, March 6 – 8 (organized by Royal Nepal Academy of Science and Technology).
Singh D and Agrawal VP (2003) Diversity of Actinomycetes of Lobuche in Mount Everest I Proceedings of International Seminar on Mountains – Kathmandu: March 6 – 8, 2002 pp. 357 – 360.
Thangadural S, Shukla SK and Anjaneyulu Y (2002) Seperation and detecrion of certain β-lactan and fluoroquinolone antibiotic drugs by thin layer chromatography. Analytical Science 18: 97-100.
Waksman SA and Henrici AT (1943) The nomenclature and classification of the actinomycetes. J Bacteriol 46: 337-341.
Williams ST and Cross T (1971) Actinomycetes. In: J.R. Norris, D. W. Robbins, (eds), Methods in microbiology, vol.4. London, 295-334, Academic Perss, NewYork.
Figure 1: Primary screening of antibiotic
produced by Lob18.2b against test organisms
Figure 2: Secondary screening of antibiotic
(crude and ethylacetate extract) against test
organisms