Drinking Water No life without water is a common saying depending upon the fact that water is one of the naturally occurring essential requirements of all life functions. It is a master solvent and all metabolic reactions of living beings depend mainly on its presence. As we know human population living in towns cities etc. depends upon municipal supplies of water. Besides a major fraction of our population which lives in rural areas especially in underdeveloped and developing countries depends upon lakes rivers ponds springs wells etc. for their water requirements.
Water is lost from the earth by way of evaporation, transpiration and exhalation and comes back to the earth by the way of precipitation. Contamination of water starts right from the beginning when water reaches the earth through air in the form of precipitations; microorganisms present in air get entry into it. After the precipitation is over and water reaches the earth surface it gets contaminated by a large array of substances such as domestic and industrial wastes i.e. sewage discharged as a consequence of civilized mans need and human and animal excrete in the form of urine and faeces.
All these contaminants deteriorate the quality of water and make it unsafe for human consumption. It is important to note that these contaminations, particularly domestic and industrial waste and faecal ones are of much biochemical concern because they not only increase the biochemical oxygen demand (BOD) but also sometime contain certain disease causing microorganisms. These disease causing microorganisms generally occur in the faeces and urine of an infected person and when discharged may gain entrance into a water supply source from where the drinking water is supplied.
This results in the transmission of disease from infected to healthy persons. However diseases transmitted via water are called water-borne disease. Each year more than 500 million people are affected with water-borne disease, and more than 10 million of them die.
Waterborne diseases
Waterborne diseases are caused by pathogenic microorganisms which are directly transmitted when contaminated drinking water is consumed. Contaminated drinking water, used in the preparation of food, can be the source of foodborne disease through consumption of the same microorganisms. According to the World Health Organization, diarrheal disease accounts for an estimated 4.1% of the total DALY global burden of disease and is responsible for the deaths of 1.8 million people every year. It was estimated that 88% of that burden is attributable to unsafe water supply, sanitation and hygiene, and is mostly concentrated in children in developing countries.
Waterborne disease can be caused by protozoa, viruses, bacteria, and intestinal parasites.
Protozoal infections
Disease and Transmission | Microbial Agent | Sources of Agent in Water Supply | General Symptoms |
Amoebiasis (hand-to-mouth) | Protozoan (Entamoeba histolytic) (Cyst-like appearance) | Sewage, non-treated drinking water, flies in water supply | Abdominal discomfort, fatigue, weight loss, diarrhea, gas pains
Fever, abdominal pain |
Cryptosporidiosis (oral) | Protozoan (Cryptosporidium parvum) | Collects on water filters and membranes that cannot be disinfected, animal manure, seasonal runoff of water. | Flu-like symptoms, watery diarrhea, loss of appetite, substantial loss of weight, bloating, increased gas, stomach |
Cyclosporiasis | Protozoan parasite (Cyclospora cayetanensis) | Sewage, non-treated drinking water | cramps, nausea, vomiting, muscle aches, low-grade fever, and fatigue |
Giardiasis (oral-fecal) (hand-to-mouth) | Protozoan (Giardia lamblia) Most common intestinal parasite | Untreated water, poor disinfection, pipe breaks, leaks, groundwater contamination, campgrounds where humans and wildlife use same source of water. Beavers and muskrats act as a reservoir for Giardia. | Diarrhea, abdominal discomfort, bloating, gas and gas pains |
Microsporidiosis | Protozoan (Microsporidia), but closely related to fungi | The genera of Encephalitozoon intestinalis has been detected in groundwater, swimming pool via AIDS patients and the origin of drinking water [2] | |
Parasitic Infections
Disease and Transmission | Microbial Agent | Sources of Agent in Water Supply | General Symptoms |
Schistosomiasis (immersion) | Schistosoma | Contaminated fresh water with certain types of snails that carry schistosomes | Rash or itchy skin. Fever, chills, cough, and muscle aches |
dracunculiasis | dracanculus medinensis | drinking water containing infective cyclops | allergic reaction,urticaria rash, nausea, vomiting, diarrhea, asthmatic attack. |
taeniasis solium | taenia solium | contaminate drinking water with eggs | intestinal disturbances, neurologic manifestations, loss of weight, cysticercosis |
fasciolopsis | fasciola | contaminated drinking water with encysted metacercaria | GIT disturbance, diarrhea, liver enlargement, cholangitis, cholecystitis, obstructive jaundice. |
hymenolepiasis nana | hymenolepis nana | contaminated drinking water with eggs | mild GIT symptoms, nervous manifestation |
hyatidosis | echinococcus granulosus | contaminated drinking water with eggs | hyatid cyst press on bile duct and blood vessels, if it ruptured cause anaphylactic shock. |
coenurosis | multiceps multiceps | contaminated drinking water with eggs | increases intacranial tension |
ascariasis | ascaris lumbricoides | contaminated drinking water with eggs | Loefflers syndrome in lung, nausea, vomiting, diarrhea, malnutrition, underdevelopment, |
enterobiasis | entrobius vermicularis | contaminated drinking water with eggs | peri-anal itch, nervous irritability, hyperactivity and insomnia |
| | | |
Bacterial infections
- Botulism - Clostridium botulinum bacteria - gastro-intestinal food/water borne; can grow in food
- Cholera - Vibrio cholerae bacteria - gastro-intestinal often waterborne
- Dysentery - Shigella/Salmonella bacteria - gastro-intestinal food/water
- Typhoid - Salmonella typhi bacteria - gastro-intestinal water/food borne. Salmonellosis - due to many Salmonella species. Water/food/direct contact borne.
Viral Infections
- Hepatitis A - Hepatitis A virus - gastro-intestinal water/food borne
- Polio - polioviruses - gastro-intestinal exposure to untreated
- Small Round Structured Virus
Allergic infections
- Hay fever - a part of disease rate is associated with the high frequency of swimming pool attendance in childhood
- Meningitis
- Trihalomethanes - a byproduct of chlorinated water which will cause bladder cancer through inhalation and dermal absorption during showering, bathing, and swimming in pools.
Potable or Non-polluted water: Water which is free of pathogenic microorganisms and chemical substances deleterious to health is referred to as potable or non-polluted water. The primary objectives for providing potable water are to make water free from harmful microorganisms and form undesirable or harmful chemicals.
Water Purification:
To be potable water must be more or less free from turbidity colour taste and odour well aerated and most important of all it must be free, from pathogenic microorganisms and harmful chemicals. Various methods of water purification are in use which depend on the mount and character of water i.e. whether the water is taken from wells springs etc. by individual families particularly in rural areas or from municipal water supply systems which serve hundred of thousand of persons in towns and cities.
Wells Springs etc. Water Purification:
Wells and springs etc. are the sources of most of the water supply for individual families in rural areas. As the water of wells and springs penetrates through the layers of soil it undergoes filtration which removes suspended particles including microorganisms. The precaution of prime importance in this regard is to ensure the supply of non-polluted water is that such water resources should strictly be located at a safe distance from possible sources of contamination e.g. pit privies cesspools septic tanks and barnyards. However the simplest and the best method to make safe for human consumption is to boil it for 10 minutes.
Municipal Water Purification:
Four main processes are employed in a municipal water purification system to ensure the supply of non-polluted drinking water. These processes are: flocculation sedimentation filtration and disinfection.

Flocculation
Water after pumping from raw water reservoir (natural sources) is collected in large tanks/basins for a sufficient time period to permit large particulate matter to settle down at the bottom. This material is removed and then the water is treated with flocculants such aluminium sulphate allum sodium alluminate colloidal silicate calcium or Bentonite which form a floc that precipitates and carriers with it microorganisms on the surface ma ns suspended organic matter settle onto the bottom of the tanks/basins. In this ways most substances that impart turbidity to water get coagulated.
Sedimentation
The water, after coagulation, is left in settling basin further for sufficient period to allow sedimentation of remaining materials. Sedimentation however considerably reduces microbial population of the water aside from removing most of the suspended particles.
Filtration
After sedimentation, water is subjected to sandfilters to remove flocks of living organisms. The process of filtration is highly critical and important as it can remove protozoan cysts and also about 98-99% of bacteria from water. The water may also be filtered through activated charcoal to remove potentially toxic organic compounds and organic compounds that impart undesirable colour and/or taster to the water.
(a) Slow Sand Filters. These sand Filters comprise of layers of fine sand, fine and coarse gravel. The floor at the base is made of tiles provided with perforations for intake of filtered water. The uppermost fine sand layer acts as a biological filter as its interspaces are clogged by colloidal floccules in water, encapsulated microorganisms, and algae, and retains bacteria making the water free from them. The efficiency of filter is further enhanced as the negatively charged bacteria are held by the positively charged colloidal material in the layer. This filter normally removes 98% of the bacterial population present in water. Nearly 5 to 7 million gallons of water per acre area of filter may be purified by this method but this method proves slow and generally requires several acres of land.
(b) Rapid Sand Filters. This method involves essentially the same equipments as the slow sand filters but the sand filter area is much smaller with provision for frequent back washing. This makes possible the rapid filtering of water. Rapid sand filters may deliver as much as 150 million gallons of water per acre/day.
Disinfection
Disinfection is the final step is municipal water purification and it ensures that no pathogenic microorganisms are carried through water. For water supplies of small towns nd localities sodium or calcium hypochlorite (NaOCl or CaOCl2 respectively) may be used to disinfect water, but for larger cities, however, chlorination (use of chlorine) has been the traditional method for disinfection. Chlorination often removes bacterial contamination since chlorine reacts with water to yield hypochlorous and hypochloric acids which are potent microbicides. Chlorine is obtained as liquid under pressure but releases into water as gas. It easily dissolves in water and, in addition to killing the microbes, it is also effective in oxidizing organic matter. However, the greater the organic matter in water, the higher the chlorine demand for a given amount of water, i.e., the chlorine demand for different waters varies depending upon the organic matter of the water. In any case, the residual amount of chlorine present in drinking water after the process of disinfection should not exceed 0.2 ppm because the disadvantage is the incidental production of trace amounts of Organochlorine compounds particularly trihalomethane (THM), a suspected carcinogen.
Fortunately, it has been found that disinfection by chloroamination is the least expensive way to reduce the formation of trihalomethane (THM) and is spreading rapidly as an alternative to chlorination. In this process monochloramine is generated directly in the water to be disinfected by adding ammonium prior to or simultaneously with chlorine or hypochlorite. Monochloramine is quite effective and produces much lower amounts of THMs.
After disinfection the water is, finally, stored in large reservoirs and is supplied for domestic consumption through gravity taps.
Determination of Water Potability
As we know that the water generally gets contaminated with pathogenic microorganisms through intestinal discharges of humans and animals (i.e., faecal contamination) and becomes an important source of water-borne diseases. It is, therefore. important that the water must be regularly analyzed to determine whether it is potable or polluted with faecal matter. Certain bacteria particularly Escherichia coil and related organisms called coliforms, faecal streptococci (e.g., Streptococcus faecalis) and Clostridium perfringens normally inhabit the large intestine of humans and other animals and are frequent present in their faces. Thus, the presence of any of these microorganisms in water provides evidence of it s being faecally polluted. If these microorganisms are traced in water, the way is also open for water-borne-disease causing pathogen since they, too, occur in faeces.
Degree of Faecal Pollution of Water
The degree of faecal pollution of water is estimated by colilitre or coli-index. The colilitre is the smallest amount of water in which one Escherichia coli is present. The coli-index is the number of individuals of E.coli found in one litre of water. Water is considered potable if the colilitre is within the limits of 300-500. It is considered to be of good quality if the coli-index is 2-3. To ensure the potability of water, i.e., whether the water sis potable or faecally polluted, generally two test techniques are employed. These techniques are : coliform-test-technique and membrane-filter-technique.
Coliform Bacteria
Coliform bacteria are the commonly-used bacterial indicator of sanitary quality of foods and water. They are defined as rod-shaped Gram-negative non-spore forming organisms. Some enteron forms can ferment lactose with the production of acid and gas when incubated at 35-37°C. Coliforms are abundant in the feces of warm-blooded animals, but can also be found in the aquatic environment, in soil and on vegetation. In most instances, coliforms themselves are not the cause of sickness, but they are easy to culture and their presence is used to indicate that other pathogenic organisms of fecal origin may be present. Fecal pathogens include bacteria, viruses,or protozoa and many multicellular parasites.
Typical genera include:
Escherichia coli (E. coli), a rod-shaped member of the coliform group, can be distinguished from most other coliforms by its ability to ferment lactose at 44°C, and by its growth and color reaction on certain types of culture media. When cultured on an EMB plate, a positive result for E. coli is metallic green colonies on a dark purple media. Unlike the general coliform group, E. coli are almost exclusively of fecal origin and their presence is thus an effective confirmation of fecal contamination. Typically, E. coli are about 11% of the coliforms in human feces.
Fecal coliforms (sometimes faecal coliforms) are facultatively-anaerobic, rod-shaped, gram-negative, non-sporulating bacteria. They are capable of growth in the presence of bile salts or similar surface agents, oxidase negative, and produce acid and gas from lactose within 48 hours at 44 ± 0.5ºC.
Fecal coliforms include the genera that originate in feces; Escherichia as well as genera that are not of fecal origin; Enterobacter, Klebsiella, and Citrobacter. The assay is intended to be an indicator of fecal contamination, or more specifically E. coli which is an indicator microorganism for other pathogens that may be present in feces.
Fecal coliforms: indicator of water quality
Basics of fecal coliform bacteria
In general, increased levels of fecal coliforms provide a warning of failure in water treatment, a break in the integrity of the distribution system, or possible contamination with pathogens. When levels are high there may be an elevated risk of waterborne gastroenteritis. Tests for the bacteria are cheap, reliable and rapid (2 -day incubation).
Potential sources of fecal coliform bacteria in water
The presence of fecal coliform in aquatic environments may indicate that the water has been contaminated with the fecal material of man or other animals. Fecal coliform bacteria can enter rivers through direct discharge of waste from mammals and birds, from agricultural and storm runoff, and from the main human sewage. However their presence may also be the result of plant material, and pulp or paper mill effluent.
Human sewage
Failing home septic systems can allow coliforms in the effluent to flow into the water table, aquifers, drainage ditches and nearby waters. Sewage connections that are connected to stormwater drainage pipes can also allow human sewage into surface waters. Some older industrial cities
Animals
Pets, especially dogs, can contribute to fecal contamination of surface waters. Runoff from roads, parking lots, and yards can carry animal wastes to streams through storm sewers. Birds can be a significant source of fecal coliform bacteria. Swans, geese, seagulls, and other waterfowl can all elevate bacterial counts, especially in wetlands, lakes, ponds, and rivers.
Agriculture
Agricultural practices such as allowing livestock to graze near water bodies, spreading manure as fertilizer on fields during dry periods, and allowing livestock watering in streams can all contribute to fecal coliform contamination.
Problems resulting from fecal contamination of water
Human health hazards
Large quantities of fecal coliform bacteria in water are not harmful, but may indicate a higher risk of pathogens being present in the water. Some waterborne pathogenic diseases that may coincide with fecal coliform contamination include ear infections, dysentery, typhoid fever, viral and bacterial gastroenteritis, and hepatitis A. The presence of fecal coliform tends to affect humans more than it does aquatic creatures, though not exclusively.
Effects on the environment
Untreated organic matter that contains fecal coliform can be harmful to the environment. Aerobic decomposition of this material can reduce dissolved oxygen levels if discharged into rivers or waterways. This may reduce the oxygen level enough to kill fish and other aquatic life. Reduction of fecal coliform in wastewater may require the use of chlorine and other disinfectant chemicals. Such materials may kill the fecal coliform and disease bacteria. They also kill bacteria essential to the proper balance of the aquatic environment, endangering the survival of species dependent on those bacteria. So higher levels of fecal coliform require higher levels of chlorine, threatening those aquatic organisms.
Bacteriological water analysis is a method of analysing water to estimate the numbers of bacteria present and, if needed, to find out what sort of bacteria they are. It is a microbiological analytical procedure which uses samples of water and from these samples determines the concentration of bacteria. It is then possible to draw inferences about the suitability of the water for use from these concentrations. This process is used, for example, to routinely confirm that water is safe for human consumption or that bathing and recreational waters are safe to use.
The interpretation and the action trigger levels for different waters vary depending on the use made of the water. Very stringent levels applying to drinking water whilst more relaxed levels apply to marine bathing waters where much lower volumes of water are expected to be ingested by users.
Plate count
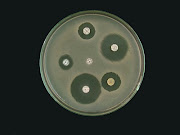
The plate count method relies on bacteria growing a colony on a nutrient medium so that the colony becomes visible to the naked eye and the number of colonies on a plate can be counted. To be effective, the dilution of the original sample must be arranged so that on average between 10 and 100 colonies of the target bacteria are grown. Fewer than 10 colonies makes the interpretation statistically unsound whilst greater than 100 colonies often results in overlapping colonies and imprecision in the count. To ensure that an appropriate number of colonies will be generated several dilutions are normally cultured.
The laboratory procedure involves making serial dilutions of the sample (1:10, 1:100, 1:1000 etc.) in sterile water and cultivating these on nutrient agar in a dish that is sealed and incubated. Typical media include Plate count agar for a general count or MacConkey agar to count gram-negative bacteria such as E. coli. Typically one set of plates is incubated at 22ºC and for 24 hours and a second set at 37ºC for 24 hours. The composition of the nutrient usually includes reagents that resist the growth of non-target organisms and make the target organism easily identified, often by a colour change in the medium. Some recent methods include a fluorescent agent so that counting of the colonies can be automated. At the end of the incubation period the colonies are counted by eye, a procedure that takes a few moments and does not require a microscope as the colonies are typically a few millimeters across.
Coliform-Test-Technique (Multiple-Tube Fermentation Test)
Coliform-test-technique (or MTFT) is a standard method followed all over the world to determine whether the water is potable or faecally polluted. The technique involves three successive steps, namely, presumptive test, confirmed test, ad completed test. In the presumptive test, lactose broth tubes are inoculated with three different water volumes to give an estimate of the most probable number green lactose bile broth in the confirmed test, and positive tubes are used to calculate the most probable number (MPN) value. Presence of coliform bacteria I water samples are established in completed tests. A general scheme of coliform-test technique is presented in.
Coliforms and Their Differentiation
Coliform is a general term used to represent aerobic, facultatively anaerobic, gram-negative, non-sporulating (non-spore forming) bacilli bacteria which produce acid and gas by fermenting lactose. Coliform bacteria were first used by U.S. Public Health Service in 1974 to assess faecal contamination
1. Negative Presumtive. The Absence of Gas in Broth Tubes Indicates Coliforms Are Absent, Incubate an Additional 24 Hours to be Sure.
2. No Gas Produced Coliform Group Absent.
3.Positive Test Gas Production; Use Positive Confirmed Tubes to Dtermine
4. Negative
5. Brilliant Green Lactose Bile Broth or Lauryl Tryptose Broth.
6. Nutrient Agar Slant
7. 24 +or- 2 Hourse 35°C
8. Negative Positive
9. After 24 Hourse of Incubation, The Tubes of Lactose or Laurly Tryptose Broth Are Examined for Gas Production.
10. Positive
11. All Positive Presumptive Cultures Used to Inoculate Tubes of Brilliant Green Lactose Bile Broth (Bglb), Incubation for 48 +or- 3 Hours AT 35°C
12. Plates of Lenives Meb Are Streaked From Positive Tubes and Incubated AT 35°C for 24+or- 3 Hours AT 35°C for 24 +or-2 Hours.
13. After 24 Hourse of Incubation, Gram-Stained Slide From the Slant is Prepared. If the Bacteria Are Gram-Negative, Nonsporing Rods and Produce Gas From Lactose, the Completed Test is Positive.
14. Coliform Colonies Are Used top Inoculate Nutrient Agar Slant and a Broth Tube.
Escherichia coli normally inhabits the intestinal tract of man and other animals, whereas Enterobacter aerogenes occurs most frequently on grains and plants but may inhabit the faeces of man and other animals. Since these two species very closely resemble each other in their morphological and cultural characteristics, they are subjected to biochemical tests for differentiation; these biochemical tests are four in number and are collectively called IMViC reactions.
IMViC Reactions
The name IMViC reactions was coined by Parr taking the first letters of each of the four biochemical tests (I = indole, M = methyl red, Vi = Voges-Proskauer reaction, and C = citrate). The reaction for a typical strain of each species of E. coli and Enterobacter aerogenes are as follows:
Test | E. coli | Enterobacter |
I. Indole (ability to produce indole) | Does | Does not |
II. Methyl red (amount of acidity produced in a special glucose-broth medium and detected by the indicator methyl-red) | Produces a pH below 4.5 which turns indicator re | Does not produce such pH and hence indicator does not turn red. It remains yellow. |
III. Voges-Proskauer reaction (ability ot produce acetylmethylcarbinol in a glucose peptone medium) | Acetylmethylcarbinol not produced | Acetylmethylcarbinol produced |
IV. Citrate (utilization of sodium citrate as source of carbon) | Does not grow in a medium in which sodium citrate is the only carbon source | Grow in a medium in which sodium citrate is the only carbon source |
Membrane-Filter-Technique
This technique used for bacteriological examination of water to determine its potability was developed in Germany during World War II and, at present, is being considered advantageous over coliform-test technique because of its significant advantages.
In this technique, a thin membrane filter-disc is used. The filter-disc consists of cellulose derivatives and can retain on its surface all bacteria from the water sample. The water is filtered through filter-disc and the disc is then transferred with a sterile forcep on to a thin absorbent pad that has previously been saturated with the appropriate medium (generally Endo-broth (GM-9) medium) and accommodated within a Petri dish. The Petri dish containing absorbent pad and filter-disc is incubated at 37°C for 18-24 hours. The medium diffuses through the pores of the filter-disc and provides nutrient to the bacteria. After the incubation is over, pone can see colonies developing upon the filter-disc. The characteristic colonies of different bacteria could now be studied to determine water potability.
Advantages
1 A large volume of water can be analyzed in a short period of time without much expenses.
2 The membrane filter-disc can be transferred from one medium to another to differentiate organisms.
3 Quantitative estimations of certain bacterial types, e.g., coliforms can be done using appropriate selective media even when the bacterial types in question are present in small numbers.
4 This technique requires much less equipment and, therefore, can be operated direct in the filed.
Disadvantages
1 High turbidity waters limit volume sampled.
2 High population of background bacteria result in overgrowth
3 Metals and phenolic compounds can absorb to filters and inhibit growth.
Sewage Microbiology
Sewage is a collective noun used to represent liquid or solid wastes carried in sewers. It consists of domestic water-borne wastes including human and animal excrete, washing waters and everything that goes down the drains of a town or a city. It also consists of industrial water-borne wastes as well as ground, surface and atmospheric waters which enter the sewerage system.
The amount of sewage produced in our country is of the order of 3.61 million cubic metres/day (about 800 million gallons/day). About 30% of the above amount comes from urban areas. It is estimated that only about 20% of one day sewage production of our country is treated and utilized, and the rest (about 80%) still remains untreated and unutilized.
Sewage Composition
The composition of sewage mainly depends upon per capita consumption of water and varies from place to place and season to season. The sewage composition can be studied under following two heads:
(a) Chemical Composition
Chemically, the sewage consists of approximately 99% water and 1% inorganic and organic matter in suspended and soluble forms. Lignocellulose, cellulose, proteins, fats, and various inorganic particulate matter exist in suspended state, whereas sugars, fatty acids, alcohols, amino acids, and inorganic ions constitute the soluble forms. However, on a average, the sewage of towns in our country contains about 350 ppm biodegradable organic matter, 52 ppm N2, 45 ppm potassium and 16 ppm phosphorus. Salts of several heavy metals such as Zn, Cr, Ni, Pb, etc. are also present above permissible levels in sewage.
(b) Microbial Composition
The microbial population per millilitre of sewage may vary from a few lacs to several millions. Various types of microorganisms, viz., microfungi, bacteria and protozoa, collectively called sewage fungus (Cooke 1954), are known to grow profusely in sewage. In addition, viruses and many microalgal genera have also been recorded from sewage. Bacteria occurring in sewage are mainly intestinal and soil inhabiting and their common types are coliforms, streptococci, clostridia, micrococci, Proteus, Pseudomonas, and lactobacilli.
Sewage Classification
Sewage may be classified mainly into two types, namely, domestic and industrial. All household wastes and human and animal excrete constitute domestic sewage, whereas the industrial wastes constitute industrial sewage. Since industrial wastes vary greatly in their composition (some may be highly alkaline such as soda wastes, some highly acidic such as acid-mine drainage, and others toxic because of presence of heavy metals, antibiotics, pesticides, etc.), the treatment of industrial sewage proves highly difficult in comparison to domestic sewage.
Characterstics of Sewage
(a) Biochemical Oxygen Demand (BOD) and Oxygen Consumption (OC) values are extremely high in sewage.
(b) The sewage organic matter undergoes anaerobic or partial decomposition resulting in the production of obnoxious gases, namely CH3, CO and H2S due to anoxic condition. Besides being toxic, these gases react with water and produce acids.
(c) Production of acids in large quantity make the sewage more acidic thus making it unfit for supporting life activities.
(d) Heavy metals are generally present in abnormal concentration in sewage.
All these characteristics of sewage, viz., condition, high acidity, high heavy metal concentration, and reduced photosynthetic rate due to poor illumination cause death of oxygen-dependent organisms such as aerobic microorganisms, plants and animals in sewage. This is the reason why sewage is dominated by organisms capable of growing in anaerobic environments.
Sewage Disposal
Sewage disposal has become of prime importance now-a-days as it brings undesirable and harmful effects on living beings. Untreated or inadequately treated sewage is generally disposed of into natural water reservoirs without taking its pros and cons into account. It is so either because we are indifferent to the consequences or because we assume that the water reservoirs are sufficiently large and so located that sewage-dilution prevents hazards. However, we can no longer rely on disposed-sewage dilution in our natural water reservoirs; the solution of sewage pollution is not its dilution. It is necessary, therefore, that the sewage must be treated before its disposal so that we can, on one hand, save organisms including men from bad effects and, on the other hand, can utilized it to the maximum for our welfare. Disposal of sewage as such or inadequately treated one, generally leads to following consequences:
Frequent dissemination of water-borne disease causing microorganisms in large number
1. Biochemical Oxygen Demand (BOD)
Biochemical Oxygen Demand (BOD; sometimes called Biological Oxygen Demand) represents the amount of dissolved oxygen needed by the microorganisms in a particular amount of water at 20°C in 5 days.
The strength of sewage is expressed in terms of BOD. The magnitude of BOD is related to the amount of organic material present in the sewage. A medium or strong sewage (having more oxidisable matter) has high BOD value, and a weak sewage (having less oxidisable matter) has low BOD value. Thus, BOD determinations provide the best information concerning the strength of sewage and the efficiency of sewage treatment processes.
Sewage BOD Determination
The BOD is determined by dilution of a measured amount of sewage with water that has been saturated with oxygen and incubation of the mixture at 20°C alongwith a control of dilution water alone. After 5 days, the residual oxygen in both control and test samples is measured by titration. The difference represents the oxygen consuming capacity of the sewage and is calculated to be expressed as parts per million (ppm) of oxygen taken up by the waste. The4 strength of the sewage in terms of pounds of BOD is calculated as follows :
=(ppm5-day BOD x gallons of sewage x 8.34)/100,000 = pound BOD
BOD effect in Domestic Water
Whenever appreciable amount of strong sewage are emptied into natural waters such as stream, ponds lakes, etc., the 7-8 ppm of free oxygen, which is normally present in water, is quickly utilized to meet out the oxygen demand of sewage. This results in a drop in oxygen level in water. When the oxygen drops below 3 ppm, the fish either leave or die. The anaerobic condition is attained and hydrolysis, putrefaction and fermentation by microorganisms follows with the result that the body of water becomes malodorous and cloudy and hence unsuited for recreational use, unfit for drinking, and other purposes.
2. Oxygen Consumption (OC)
OC represents the amounts of dissolved oxygen utilized by biotic communities for the oxidative decomposition of organic matter in one liter of water in one hour. Dissolved oxygen in sewage becomes totally depleted because of high oxygen demand and low photosynthetic rate. Photosynthesis in sewage comes down because of poor illumination as the suspended solids obstruct sun light. However, the depletion of dissolved oxygen leads to anoxic (oxygenless) condition.
Depletion of dissolved oxygen in water leading to anoxic (oxygenless) condition which may ultimately kill O2 dependent aquatic life.
Creation of offensive odour and debris-accumulation due to which value of property decreases.
Increased danger of swimming in water and diminished value of water for other recreational purposes.
Sewage Treatment
Objectives
Our objectives behind the sewage treatment would be to kill pathogenic microorganisms prevent anoxia, raise the pH to alkaline side, increase photosynthetic rate, reduce organic content, etc. When these objectives are achieved by the way of treating the sewage, the conditions prevailing in a natural water reservoir are induced in sewage water and the latter can be reused.
Sewage Treatment Processes
Sewage treatment processes are many and varied. We will discuss only those sewage treatment processes which are generally applied in single dwelling unit situations and municipal situations.
Single Dwelling Unit Treatment Processes
(a) Outdoor Toilets
Where plumbing installations cannot be undertaken for any reason, the toilets or water closets may be constructed outdoors. While this arrangement is undertaken, care could be taken to see that flies have no access to these and changes of drainage from these, joining water supplies, are eliminated.
(b) Septic Tanks
These are used for residential quarters. All the residential sewage is passed through suitable pipes leading to a tank located to a tank at a suitable place and made of metal or concrete. The heavy particles of sewage settle down and undergo anaerobic decomposition whereas the gases and clear water are allowed to go out through perforated pipes ramified within the ground. The septic tank device should be so fitted that the sewage does not drain by any chance into water supply of the residence. The sludge in the tanks must be periodically removed to prevent clogging of the pipes.

1. House | 4. Sludge | 7. Distribution Box |
2. Inlet | 5. Septiv Tank | 8. Perforated Pipe |
3. House Sewerline | 6. Outlet | 9. Absorption Field |
(c) Imhoff Tank
This is fact, a modification of septic tank and is generally used to treat larger community sewage. It consists of two chambers, one above the other. The top chamber receives sewage and the heavier particles settle into the lower chamber and slowly decompose under anaerobic conditions. The gas liberated (mainly methane) can be drawn out through a passage and utilized as fuel. The sewage effluent (remaining sewage water) is either let into larger body of water, or is subjected to aerobic decomposition. The sludge is periodically removed, aerated and used as manure.

1. Gas | 2. Sludge |
3. Sludge Pipe | 4. Gas |
5. Flow Chamber | 6. Digestion Chamber |
Municipal Treatment Processes
Municipal sewage treatment systems carry out various steps involved. These steps are, namely primary (or mechanical) treatment, secondary (or biological) treatment, and tertiary (or final) treatment.
A. Primary (or Mechanical) Treatment
When the sewage arrives at a sewage treatment plant, it is first subjectd to mechanical (or physical) means. viz., flowing dilution and sedimentation to remove its coarse solid materials. The sewage is passed through a series of filters of graded opening and then allowed to flow through sedimentation units (tanks, basins, etc.). Coarse solid materials are concentrated in and collected from sedimentation units; these particulate materials are collectively called sludge. Following sedimentation, the sludge and liquid affluent are processed separately during secondary treatment.
B. Secondary (or Biological) treatment
This is purely a biological treatment of mechanically treated sewage and concerns microbial activity which biodegrades organic substrates and oxidisable inorganic compounds. This treatment accomplishes two important phases, namely, aerobic phase and anaerobic phase. The aerobic phase consists of aerobic digestion of sludge by various filters (e.g., trickling filters), oxidation ponds and activated sludge process, and the anaerobic phase is represented by anaerobic digestion of sludge.
I. Aerobic Phase of Secondary Treatment
(a) Aerobic Digestion in Trickling Filters. Tricking filter consists of generally 6-10 feet deep bed of crushed stone, gravel, slag, or similar material. The sewage effluent is sprayed over the surface of the bed; the spraying saturates the effluent with oxygen.

1. Raw Sewage | 7. Anaerobic digestion of sludge in Anearobic Digestion Tank | 13. Aerobic microbes break down organic matter to CO2 and H2O |
2. Grit Chamber | 8. Anaerobic Phase | 14. Sedimentation Tank |
3. Sedimentation Tank | 9. Aerobic Phase | 15. Chemical treatment for nitrate and phosphate, etc. |
4. Anaerobic microbes break down organic matter into soluble substances and gas-mixture (CH4,CO2,H2N2) | 10. Sewage effluent (liquid) | 16. Chlorination |
5. Gas mixture may be used to operate sewage plant or used as fuel | 11. Sedimentation tank | 17. Clean water |
6. Sludge (solid) | 12. Aerobic digestion of remaining sludge in effluent in Activated Sludge Digester or Trickking Filter |
The bed surface becomes coated with aerobic microbial flora consisting of microalgae, microfungi, bacteria, and protozoa. As the effluent seeps over, the aerobic microbes degrade the organic matter. However, the treated effluent collected at the bottom of the tank is passed to sedimentation tank and, like activated sludge process, the effluent follows tertiary. Aerobic digestion of sewage organic matter in a trickling filter is a very slow process.
(b) Oxidation Ponds. Oxidation pond sewage-treatment is recommended for small communities in rural areas where suitable and sufficient land is available. Oxidation ponds (also called Lagoons or Stabilization Ponds) are generally 2-5 feet deep shallow ponds designated to allow direct wind action and algal growth on the sewage effluent. Oxygen supplied from air and produced as a result of algal photosynthesis fulfils biochemical oxygen demand (BOD) of sewage effluent and thus helps in maintaining aerobic condition in sewage effluent. In such condition the aerobic microbes grow rapidly and digest organic matter. Chlorella pyrenoidosa is a common algal representative grown in oxidation ponds.
(c) Activated Sludge Process. In this process, the mechanically treated sewage effluent (serge liquid) is pumped into a sedimentation or settling tank wherein the sewage flocs and settle out. A portion of sewage ‘flow’ is returned to activate a new batch of mechanically treated sewage effluent, and the rest is pumped to activated sludge digester where air is blown by several jets. Thus, in the presence of plentiful oxygen, oxidation of sewage effluent is brought about by aerobic microorganisms which break down organic matter to CO2 and H2O. Now the effluent is passed through a sedimentation tank. Though about 90% of the organic matter of the effluent is digested via this process, the effluent still contains considerable amount of nitrate and phosphate, etc. It is therefore, not safe to discharge effluent at this stage into a large body of water as both nitrate and phosphate can cause eutrophication. Now the effluent, which looks clear at this stage, is subjected to tertiary (final) treatment for further purification.
Activated Sludge
When we vigorously serate sewage the finely suspended and colloidal material (including microbes) of it forms aggregates called floccules. These floccules collectively result in the formation of sewage floc. When this sewage floc is sedimented and then inoculated to a fresh vigorously aerating sewage, the floccule formation in the latter takes palce in shorter time duration than the previous one.
As a result of the repetition of this process, (i.e., inoculation of sedimented floc to a fresh aerating sewage, sedimentation, inoculation of sedimented floc again to a fresh aerating sewage, etc.) a stage is reached where complete flocculation of a fresh sewage takes place in very short time duration, e.g., a few hours. These particles of sedimented floc are called activated sludge and consist of large number of very actively metabolizing bacteria, yeasts, molds, and protozoa. The use of activated sludge is of great significance in biological treatment of sewage as it reduces aeration period of sewage to 4-8 hours.
II. Anaerobic Phase of Secondary Treatment
C. Tertiary (or Final) Treatment
Since the sewage-effluent treated during secondary treatment process still contains non biodegradable organic pollutants (if sewage contains industrial wastes) and mineral nutrients particularly nitrogen and phosphorus salts, it is subjected to tertiary (or final) treatment for their removal. If not so, the sewage effluents containing nitrogen and phosphorus salts can cause serious eutrophication in aquatic ecosystems. Nonbiodegradable organic pollutants are normally removed by using activated carbon filters whereas phosphorus and nitrogen salts by chemical treated. Phosphorus salts are precipitated by liming and the nitrogen present mainly as ammonia is removed by volatilization (vigorous aeration at elevated temperature) at a high pH. These treatments result in a high-quality effluent which does not cause eutrophication.
The find step of tertiary treatment is disinfection which is commonly accomplished by chlorination using either sodium or calcium hypochlorite (NaOCl or CaOCl2 respectively) or chlorine. Now the effluent is a clean water and is considered microbiologically safe even for human consumption.
USEPA testing requirements
The new United States Environmental Protection Agency (EPA) Total Coliform Rule (TCR) imposes major monitoring changes for the drinking water industry. The testing requirements for drinking water under the new TCR are markedly increased and thus, more thorough. Not only is the number of routine coliform tests increased, especially for smaller utilities, but the new regulation also requires automatic repeat testing from all sources that show a total coliform positive (known as triggered source water monitoring).
The current EPA recommendations for body-contact recreation is fewer than 100 colonies/100 mL; for fishing and boating, fewer than 1000 colonies/100 mL; and for domestic water supply, for treatment, fewer than 2000 colonies/100 mL. The drinking water standard is less than 1 colony/ 100 ml.







 Schematic representation of Phosphorous cycle
Schematic representation of Phosphorous cycle