Table 1: List of malaria vaccines
|
Type of Vaccines
|
Name:
Compositions
|
Beneficial / Limitations
|
|
Pre-erythrocytic vaccines
|
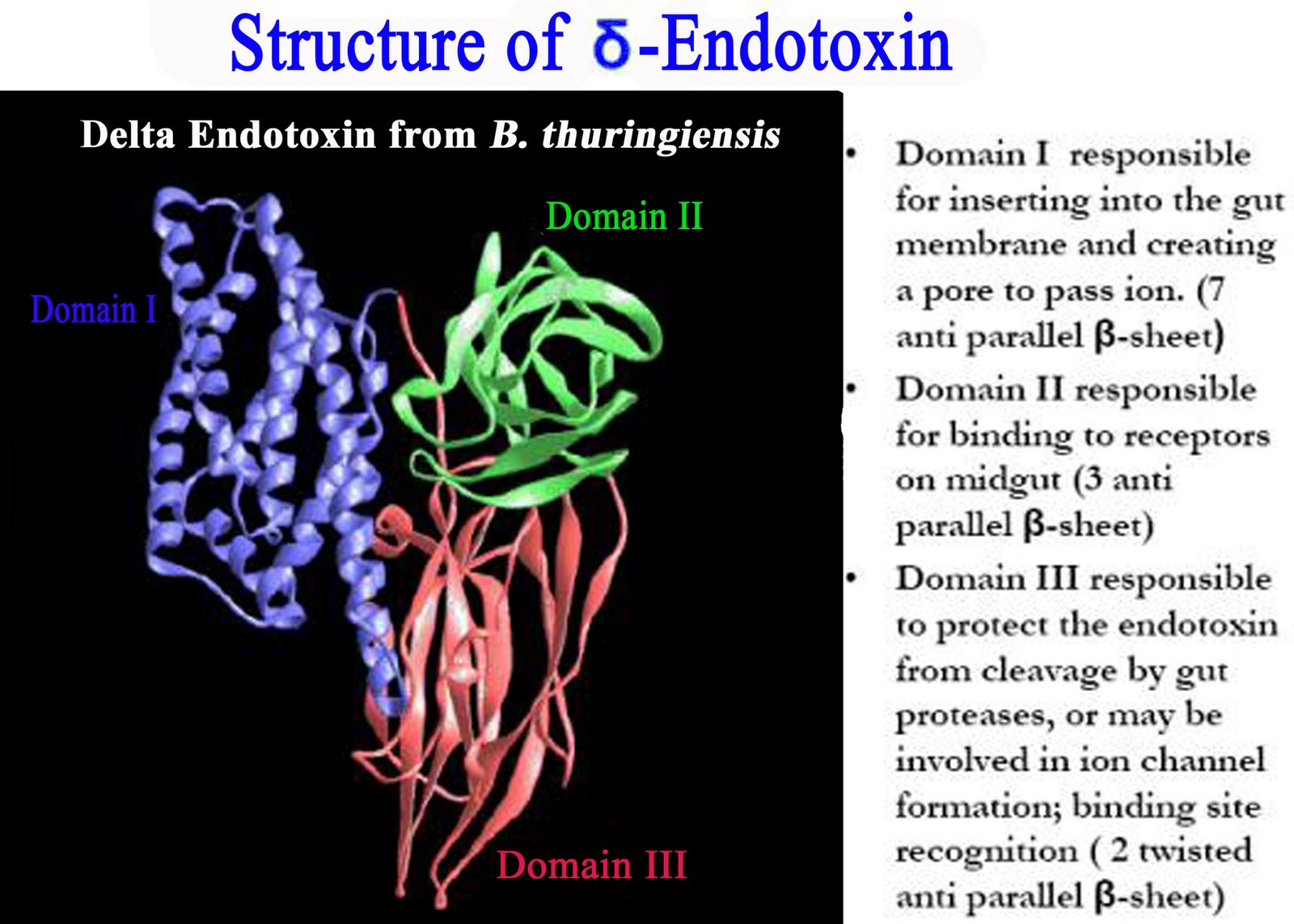
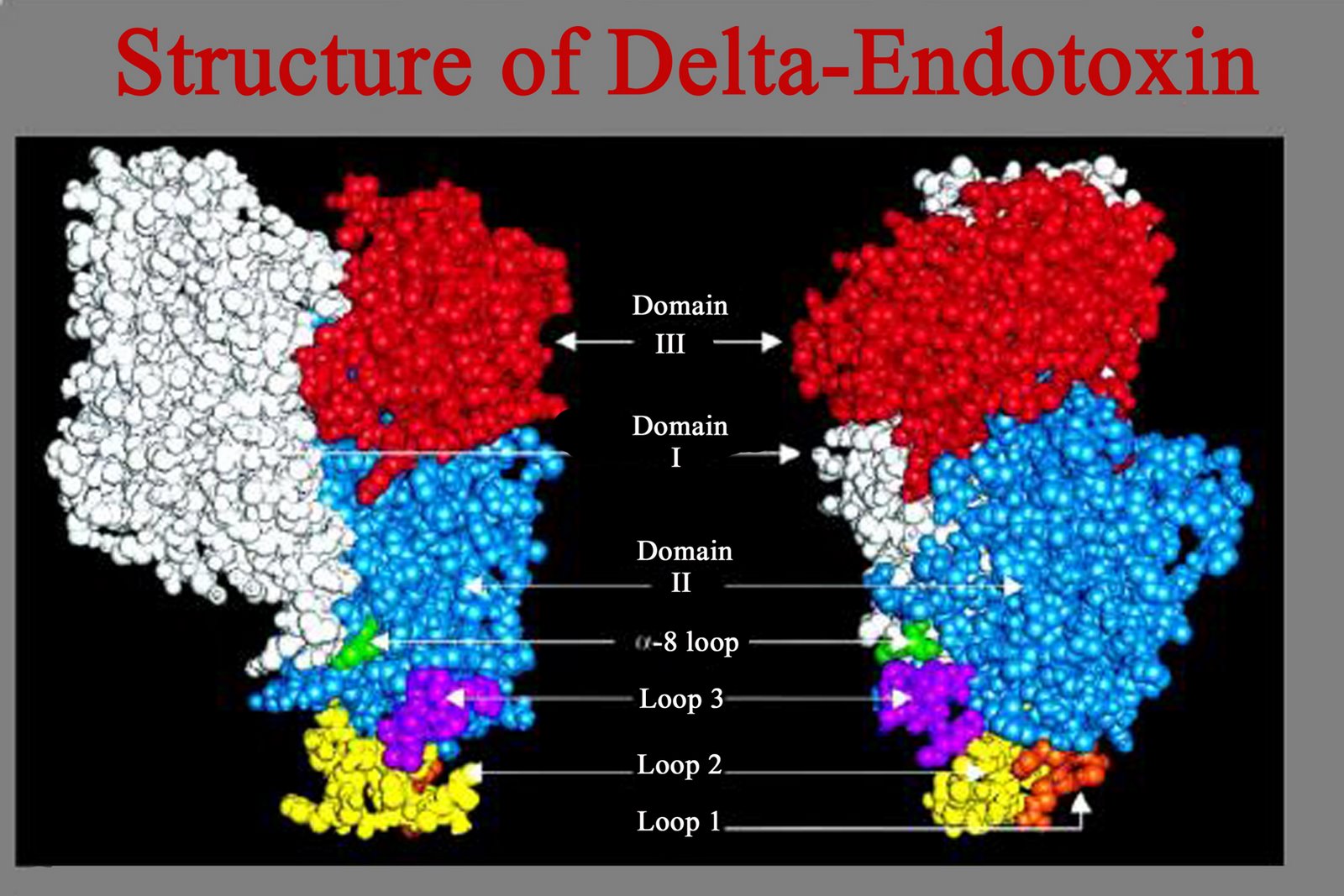
1. RTS,S/AS01E
·
Adenovirus (Ad35) vectored
circumsporozoite (CS) protein in prime boost with RTS,S/AS01E
·
Multiple epitope constructs
(ME-TRAP); a pre-erythrocytic fusion antigen consisting of 17 B-cell, CD4+,
CD8+ T cell epitopes of 6 Pf antigens fused to the T9/96 allele of TRAP
(Thrombospondin related adhesion protein)
|
·
Only the vaccine in Phase 3
clinical trial
·
Low efficacy but decreases
mortality
|
|
2. AdCh63/MVA ME-TRAP
·
Simian Adenovirus (Ad35) encoding
ME-TRAP boosted with modified vaccinia virus Ankara (MVA)
|
·
Long lasting CD8+ T cell
responses
|
|
3. Polyepitope DNA EP1300
·
DNA vaccine includes multiple
epitopes with linker sequences from four pre-erythrocytic antigens CS,
SSP2/TRAP, liver stage antigen-1 (LSA-1) and Exported protein-1 (EP-1)
|
·
Immunogenicity is not enhanced in
human as compared to animals
|
|
4. PfSPZ
·
Metabolically active
non-replicating malaria sporozoite vaccine (Pf sporozoites prepared by
thawing from liquid nitrogen)
|
·
Not protective but need to
revised in terms of dose and route of administration
|
|
5. Genetically Attenuated sporozoites-GAS
|
·
Safe and immunogenic
|
|
6. FP9 CS/MVA CS
·
Attenuated fowl pox strain (FP9)
expressing CS protein boosted with MVA coding CS protein
|
·
Didn’t boost the T-cell response
|
|
7. DNA CS/MVA CS
·
Plasmid DNA encoding CS protein
replace fowl pox strain (FP9) boosted with MVA coding CS protein
|
·
Modest T-cell response
|
|
8. RTS,S/AS02 + MVA CS
·
Boosting RTS,S/AS02 with MVA
encoding CS
|
·
Greater CMI response
·
|
|
9. CS DNA Immunization or VCL-2510
·
Gene for full length CS protein
in Plasmid DNA
|
·
No anti-CS antibodies
|
|
10. MUST DO5
·
Multi-Stage DNA vaccine operation
5 antigens
·
5 antigens; CS, SSP2/TRAP, LSA-1,
LSA-3 and EP-1 adjuvanted with GM-CSF (Granulocyte Macrophage colony
stimulating factor)
|
·
No efficacy
|
|
11. DNA CS / RTS,S/AS02
·
DNA vaccine VCL-2510 containing
full length CS gene and adjuvanted with RTS,S/AS02
|
·
No significant improvement than
using alone
|
|
12. RTS,S/AS02 TRAP
·
Combination of CS with TRAP
|
·
Improve efficacy
|
|
13. HepB Core Ag CS VLP
·
Also called ICC-1132 or
Malariavax
·
Hepatis B core antigen modified
to include B-cell and 2 CD4+ epitopes of CS protein
|
·
No efficacy to sporozoites
|
|
14. PfCS102
·
Chemically synthesized Pf CS
protein with C-terminus (Amino acids 282-383)
|
·
Don’t reduce parasitemia
|
|
15. FP9/MVA polyprotein
·
Prime Boosting with FP9 and MVA
viruses with six antigens polyprotein (STARP, TRAP, LSA-1, LSA-3, Pfs16 and
EP-1)
|
·
Low efficacy with sporozoite challenge
|
|
16. FMP011/AS01B
·
LAS-1 E. coli expressed
evaluated with AS01B and AS02A
|
·
Associated with acute coronary
syndrome
|
|
|
|
Blood Stage (Erythrocytic)
vaccines
|
1. AdCh63/MVA MSP-1
·
Pf Merozoite Surface Protein-1
(MSP-1) expressed and boosted with AdCh63 and MVA vectors
|
·
Low IgG response
|
|
2. FMP010/AS01B
·
E. coli expressing FVO allele 42kD C terminus of MSP-1 and
adjuvants AS01B
|
·
Good immunogenicity but
inadequate clinical efficacy
|
|
3. FMP2.1/AS02A
·
FMP2.1, in which E. coli
expressed Apical membrane antigen (AMA-1) and adjuvant AS02A
|
·
Strong Th-2 biased response
|
|
4. FMP2.1/AS01B
·
FMP2.1, in which E. coli
expressed Apical membrane antigen (AMA-1) and adjuvant AS01B
|
·
Adverse effect (rashes after 18
days of vaccination)
|
|
5. AMA-C1/Alhydrogel + CPG 7909
·
Pichia pastoris expressed Apical membrane antigen (AMA-1) with both
FVO and 3D7 strains and adjuvants Alhydrogel + CPG 7909
|
·
Reduction of hemoglobin
·
|
|
6. AdCh63 AMA-1/MVA AMA-1
·
Apical membrane antigen-1 (AMA-1)
expressed and boosted with AdCh63 and MVA vectors
|
·
CD4+ responses are higher than
CD8+ response
|
|
7. EBA175RII
·
Erythrocyte binding antigen (EBA)
in merozoites and highly conserved region of Pf
|
·
Safe and immunogenic
|
|
8. SERA5
·
Blood stage antigen in
trophozoites and schizonts (SE36 expressed in E. coli acts as fragment
of SERA5 antigen)
|
·
Safe and 100% seroconversion
|
|
9. BSAM-2/Alhydrogel +CPG
·
combination vaccine including MSP1 and
AMA1 components. It contains a mixture of recombinant proteins with equal
parts P. pastoris expressing FVO and
3D7 strains of AMA1 and E. coli expressing the FVO and 3D7 strains of MSP1.
|
·
Under evaluation
|
|
10. JAIVAC
·
This combination vaccine consists of MSP1
and EBA175, each of which is an E. coli expressed recombinant protein adjuvanted with Montanide
ISA 720
|
·
Synergistic effects
|
|
11. GMZ2
·
L. lactis expressed recombinant
fusion protein of glutamate rich protein (GLURP) and MSP-3 adjuvanted with
Al(OH)3
|
·
Acceptable safety
·
Both IgG and memory B cell
response
|
|
12. Combination B (RESA, MSP-1, MSP-2)
13. FMP1/AS02A
14. MSP-1-C1/AlOH/AlOH + CPG
15. MSP2-C1/ISA720
16. AMA1-C1/ISA720
17. AMA-FVO
18. PfCP2.9
|
·
Already terminated (No succeed)
|
|
|
|
|
|
Gametocyte (Transmission
Blocking) Vaccines
|
1. Pfs25
·
Ookinete surface protein Pfs25
·
The antigen preparations including ookinete surface protein Pfs25, and the gametocyte
antigens Pfs48/45 and Pfs230 are used in the TBV vaccines. Pfs25 was the first antigen to progress clinically but the reactogenicity
was found to be very low.
|
·
Low reactogenicity
|
|
2. Pfs48/45 and Pfs230
·
Contain the gametocyte antigens
Pfs48/45 and Pfs230
|
·
Low reactogenicity
|
|
|
|
|
|
Combined Vaccines
|
1. NMRC-M3V-D/Ad-PfCA Prime/Boost and NMRC-M3V-Ad-PfCA
·
DNA based vaccine containing CS
antigen with AMA-1 antigen boosted with Adenovirus-5-vectors
|
·
Increase CD8+ T cell response but
no sterile protection
|
|
2. CS AMA1 Virosomes
·
Synthetic peptide vaccines
·
Phosphatidylethanolamine (PE)
conjugates on the surface of immunopotentiating reconstituted influenza
virosomes (PEV301 and PEV302)
|
·
No sterile protection
|
|
3. SPf66
·
Synthetic polypeptide vaccine
consisting of CS and merozoites protein1 (MSP1) epitopes adjuvanted with alum
|
·
Low vaccine efficacy
|
|
4. RTS,S/AS02 (CS) and FMP-1/AS02 (MSP1)
·
Mixing RTS,S/AS02 (CS) and
FMP-1/AS02 (MSP1) with both CS and MSP1 antigens
|
·
FMP1 gave no protection in the
challenge model
|
|
|
|
|
|
P. vivax Vaccines
|
1. PvCSP
·
Circumsporozoite protein from P.
vivax
|
·
Under clinical trails
|
|
2. ChAd63-MVA PvDBP RII and PvDBP RII/GLA-SE
·
Extracellular, cysteine-rich
region II (P. vivax Duffy-binding protein; PvDBP_RII)
|
·
Under clinical trails
|
References:
Artaud C, Kara L, and Launay O. Vaccine
Development: From Preclinical Studies to Phase 1/2 Clinical Trial (Chapter 12).
Frederic Ariey et al. (eds.), Malaria Control and
Elimination, Methods in Molecular Biology, vol. 2013, © Springer Science + Business Media, LLC, part of Springer
Nature 2019
Bejon P, Lusingu J, Olotu A, et al. Efficacy of
RTS,S/AS01E Vaccine against Malaria in Children 5 to 17 Months of Age. N Engl J
Med. 2008 December 11; 359(24): 2521–2532.
Carvalho LJM, Daniel-Ribeiro CT and Goto H. Malaria Vaccine:
Candidate antigens, Mechanisms, Constraints and Prospects. Acand. J. Immunol.
56, 337-343, 2002.
Draper SJ, Sack BK, King
CR, Nielsen CM, Rayner JC, Higgins MK, Long CA and
Seder RA. Malaria
Vaccines: Recent Advances and New Horizons. Cell Host & Microbe 24, July 11, 2018.
2018: 43-56.
Frimpong A, Kusi KA, Ofori MF and Ndifon W. (2018). Novel Strategies for Malaria
Vaccine Design. Front. Immunol. 9:2769. doi: 10.3389/fimmu.2018.02769
Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, et al. Genome
sequence of the human malaria parasite Plasmodium falciparum. Nature (2002)
419:498.
Karunamoorthi K. Malaria
vaccine: A future hope to curtail the global malaria burden. Int J Prev Med
2014; 5: 529-38.
Longley
RJ, Hill AVS and Spencer AJ (2015) Malaria vaccines: identifying Plasmodium
falciparum liver-stage targets. Front. Microbiol.6:965. doi:10.3389/fmicb.2015.00965
Moreno A and Joyner C. Malaria vaccine clinical trials:
what’s on the horizon. Curr Opin Immunol. 2015 August; 35:
98–106.
Ockenhouse CF, Regules J, Tosh D, Cowden J, Kathcart A,
Cummings J, et al. (2015). Ad35.CS.01 - RTS,S/AS01 Heterologous Prime Boost
Vaccine Efficacy against Sporozoite Challenge in Healthy Malaria-Naïve Adults.
PLoS ONE 10(7): e0131571. doi:10.1371/journal.pone.0131571
RTS,S Clinical Trials Partnership.
Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster
dose in infants and children in Africa: final results of a phase 3,
individually randomized, controlled trial. Lancet. 2015 July 04;
386(9988): 31–45.
Salamanca DR, Gómez M, Camargo A, Cuy-Chaparro L, Molina-Franky
J, Reyes C, Patarroyo MA and Patarroyo ME (2019). Plasmodium falciparum Blood Stage
Antimalarial Vaccines: An Analysis of Ongoing Clinical Trials and New
Perspectives Related to Synthetic Vaccines. Front. Microbiol. 10: 2712.
Schwartz L, Brown GV, Genton B and Moorthy VS. A review of
malaria vaccine clinical projects based on the WHO rainbow table. Malaria
Journal 2012, 11:11
Stewart VA, McGrath SM, Dubois PM, Pau MG, Pascal M,
Shott J, Cobb M, Burge R, Larson D, Ware LA, Demoitie M, Weverling GJ, Bayt B,
Custes JHHV, Dubois M, Cohen J, Goudsmit J, and Heppner DG. Priming with
an Adenovirus 35-Circumsporozoite Protein (CS) Vaccine followed by RTS, S/AS01B
Boosting Significantly Improves Immunogenicity to Plasmodium
falciparum CS Compared to That with Either Malaria Vaccine Alone. American
Society for Microbiology May 2007; 75 (5): 2283–2290.
Stewart VA, McGrath SM, Walsh DS, Davis S, Hess AS, Ware
LA, Kester KE, Cummings JF, Burge JR, Voss G, Delchambre M, Garcon N, Tang DB,
Cohen JD, Heppner DG. Pre-clinical evaluation of new adjuvant formulations to
improve the immunogenicity of the malaria vaccine RTS, S/AS02A. Vaccine 24: (2006): 6483–6492.
WHO, Background paper on the RTS,S/AS01 malaria vaccine-2015.