Microorganisms which play a significant role in the operation of nitrogen cycle may be divide into four major groups :
Group 1: Those microorganisms which are capable of fixing atmospheric nitrogen (biological nitrogen fixation), i.e., of combining free nitrogen with other elements or compounds.
Group 2: Those microorganisms which bring about the production of ammonia, i.e., ammonification
Group 3: Those microorganisms which oxidise ammonia to nitrite and nitrite to nitrate, i.e., nitrification.
Group 4: Those microorganisms which are capable of transforming nitrates to nitrogen or nitrous oxide, i.e., denitrification.
Fixation of Free Nitrogen (Biological N2-fixation)
A large number of microorganisms are known to have the ability to reduce atmospheric nitrogen into nitrogenous compounds. This conversion of molecular (atmospheric) nitrogen into nitrogenous compounds by microorganisms is called ‘biological fixation’
Ammonification
Proteins and nucleic acids of the dead remains of plants and animals, and the excretory products of animals are degraded by microorganisms with the liberation of ammonia. This process is called ‘ammonification’. Two different steps are involved in ammonification:
(b) Amino acid degradation
(a) Proteolysis
Proteins=/proteses/=Peptides=/peptidase/=Amino acids
Schematic Representation of Nitrogen Cycle

b)) Amino Acid Degradation
The amino acids now undergo degradation by microbial attack. They are deaminated (i.e, removal of the amino group) to yield ammonia. Microorganisms exhibit several variations of deamination reactions in soil; one of the end products is always ammonia. Example-
Nitrification
Ammonia produced by the degradation of manures and organic matter may not be directly available even to those plants which can use it, for it is readily leached from soil and is usually converted to nitrate with the help of certain microorganisms. This conversion (oxidation) of ammonia to nitrate is called ‘nitrification’. Nitrification is carried out in two stages by specific bacteria:
(a) Oxidation of Ammonia to Nitrate
2NH3 + 3O2 = 2HNO2 + 2H2O
Nitrosomonas are the most important agents of oxidation of ammonia to nitrite in soil. In addition certain other bacteria, e.g., Nitrosococcus, Nitrosospira, Nocardia and Streptomyces have been known to oxidize NH3 to nitrite.
(b) Oxidation of Nitrate to Nitrate
HNO2 + 1/2O2 = HNO3
This oxidation of nitrate to nitrate in soil is dependent on the activities of bacteria belonging mainly to the genera Nitrobacterium. In addition certain fungi e.g., Cephalosporium, Aspergillus and Penicillium have been reported able to carry out nitrification were discovered to be a biological process by Schloesing and Muntz (1877); Winogradsky isolated the bacteria responsible for biological nitrification in 1890.
Denitrification
The transformation (reduction) of nitrates gas or nitrous oxide by certain microorganisms is called ‘denitrification’. The process depletes the soil of an essential nutrient, the nitrogen for plant growth and, therefore, is not desirable. Some important microorganisms responsible for this process are Thiobacillus denitrificans, Micrococcus denitrificans and some species of Serratia, Pseudomonas, Bacillus, Achromobacter, and Paracoccus. The process of denitrification is completed by means of various steps in presence of ‘reductase’ enzymes. The overall reaction is as follows:

Denitrification does not occur to any significant degree in well aerated soils with moderate amounts of nitrates and organic matter. It occurs seriously in water logged anaerobic soils with high organic matter content.
CARBON CYCLE
Carbon is the most important element in the biological system and constitutes about 50% of all living organism. Carbon dioxide present in the atmosphere or dissolved in water is the ultimate source of organic carbon compounds occurring in nature; its complete cycle is schematically represented. The cycle of carbon in nature comprises of two main processes:
(i) The conversion of oxidized form of carbon into reduced organic form by photosynthetic organism
(ii) Restoration of original oxidized form through mineralization of the organic form by the microorganisms.
Conversion of oxidized form of Carbon (CO2) into Reduced Organic Form
CO2 is reduced into organic carbon compounds mainly by the process of photosynthesis. Photosynthesis algae and higher plants are the most important agents of carbon dioxide fixation. In the ocean the major plant forms that fix carbon are the free floating microscopic algae called phytoplankton. They are estimated to fix annually about 1.2 x 1010 tons of carbon. Nearly 1.6 x 1010 tons of carbon is said to be fixed annually by photosynthetic terrestrial plant life. Besides, autotrophic and heterotrophic bacteria are also capable of synthesizing organic metter from inorganic carbon. I addition to the occurrence of photosynthesis among microorganisms, the latter also represent the example of CO2 fixation into organic compounds which are as follows:
(a) The carbon dioxide represents the sole source of carbon for autotrophic bacteria. The latter fix CO2 to carbohydrates by a reduction reaction.
CO2 + 2H2 = (CH2O)x + H2O
(b) Heterotrophic bacteria fix carbon dioxide commonly.
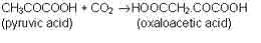
Schematic Representation of Carbon Cycle

Restoration of Original Oxidized From (CO2) through Mineralization of the Organic Forr.
One can consider three different modes through which the organic matter is mineralized and the CO2 is released in the atmospheric. They are:
(a) Process of respiration
(b) Accidental (forest fire) and intentional fuel burning
(c) Decomposition of organic matter by microorganisms.
The process of respiration in plants and animals, and the accidental and intentional burning or plants and their parts result in the breakdown of organic carbon compounds releasing carbon dioxide in the atmosphere.
Decomposition of Organic Matter by Microorganisms
The organic carbon compounds that eventually are deposited in the soil are degraded by the activities of microorganism which are mainly the bacteria and fungi. The CO2 is released into the air and soil.
(i) Cellulose Decomposition
Cellulose is the most abundant organic material in plants. It is readily attacked by many species of fungi and bacteria. The process of cellulose decomposition to carbon dioxide can be summarized in the form of following reactions.
The fungi which decompose cellulose in soil are mainly Trichoderma, Aspergillus, Pencillium, Fusrium, Chaetomium, Verticillium, Rhizoctonia, Myrothecium, Merulius, Pleurotus, Fomes, etc.
The bacteria that brings about cellulose decomposition in soil consist mainly of the species of Clostridium, Cellulomonas, Streptomyces, Cytophaga, Bacillus, pseudomonas, Nocardia, Micro-monospora, Sporocytophaga, Polyangium, Cellfalcicula, etc
(ii) Hemicellulose Decomposition
Hemicelluloses are the polymers of simple sugars such as pentoses, hexoses and uronic acid. The decomposition of hemicellulose by microorganisms takes places through the agency of extracellular enzymes called hemicellulases.
The fungi that degrade hemicelluloses in soil are exemplified by Chaetomium, Aspergillus, Pencillium, Trichoderma, Fusarium, Humicola, etc.
Bacillus, Pseudomonas, Cytophaga, Vibrio, Erwinia, Streptomyces, Actinomyces, etc. are the bacteria that degrade hemicelluloses in soil.
(iii) Lignin Decomposition
Lignin is the third most abundant constituent of the plants. It is highly resistant of microbial degradation. However, certain fungi (exemplified by Aspergillus, penicillium, Fusarium, Lenzites, Clavaria, Polyporus, etc.) are known to degrade lignin at slow rates.
Sulphur like nitrogen and carbon, is an essential part of all living because sulphur containing amino acids are always present in almost all kinds of proteins. Plants can absorb directly the sulphur containing amino acids, e.g., cystine, cysteine and methionine but these amino acids fulfil only a small proportion or requirements for sulphur. To fulfil rest of the requirements of plants, sulphur passes through a cycle of transformation mediated by microorganisms. It accumulates in the soil mainly as a constituent of organic compounds and has to be converted to sulphates to become readily available to the plants. The complete cycling of sulphur is schematically represented and some important steps are discussed as under:
(a) Degradation of Organic Compounds to Release H2S
(i) Degradation of proteins (proteolysis) liberates amino acids which generally contain sulphur.
Protein =/degradation/=Amino acid
Schematic Represenation of

|
(ii) Enzymatic activity of many heterotrophic bacteria results in the release of H2S from further degradation of sulphur containing amino acids.
Example
(iii) Sulphates may also be reduced to H2S by the action of Desulfotomaculum bacteria. Example
CaSO4 + 4H2 =/ Desulfotomaculum /= Ca(OH)2 + H2S + 2H2O
(b) Oxidation or Hydrogen Sulphide (H2S) to Elemental
Hydrogen sulphide undergoes decomposition to produce elemental sulphur by the action of certain photosynthetic sulphur bacteria, e.g., members belonging to the families Chlorobiaceae (Chlorobium) and Chromatiaceae (Chromatium). Example:

Some non-sulphur purple bacteria, e.g., Rhodospirillum, Rhodopseudomonas, and Rhodomicrobium which are facultative phototrophs and grow aerobically in the dark and anaerobically in the light can also degrade H2S to elemental sulphur
(c) Oxidation of Elemental sulphur to Sulphates
Elemental form of sulphur accumulated in soil by earlier described processes cannot be utilized as such by the plants. It is oxidized to sulphates by the action of chemolithotrophic bacteria of the family Thiobacteriaceae (Thiobacillus thiooxidans). Example:

Sulphates are the compounds that can readily be taken by the plants and are beneficial to agriculture in the following three ways:
1. It is the most suitable source of sulphur and is readily available to plants.
2. Accumulation of sulphates solubilizes organic salts that contain plant nutrients such as phosphates and metals
3. Sulphate is the anion of a strong mineral acid (H2SO4) and prevents excessive alkalinity due to ammonia formation by soil microorganisms.
Sulphate is assimilated by plants and is incorporated into sulphur amino acids and then into proteins. Animal fulfil their demand or sulphur by feeding on plants and plant products.
d) Reduction of Sulphates
Sulphate is first reduced to H2S by sulphate reducing microorganisms under anaerobic conditions. Many bacteria including species of Bacillus, Pseudomonas, Desulfovibrio do this work. The mechanism of sulphate reduction to hydrogen sulphide involves, firstly, the reduction of sulphate to sulphite utilizing ATP and, secondly, reduction of sulphite to hydrogen sulphide. The whole mechanism of the reduction of sulphate to hydrogen sulphide by Desulfovibrio desulfuricans, the most important bacterium of this reduction, can be represented as follows:

PHOSPHORUS CYCLE
Phosphorus is one of the most important constituents of several important compounds always present in organism. It occurs both in organic (nucleic acids, nucleopoteins, phospholipids, etc.) and inorganic (phosphate) forms in the living organisms. Animals possessing bones have large amount of phosphorus in its inorganic form. However, phosphorus is added to soil through chemical fertilizers, excrete and organism-resides. Though there is plenty of phosphorus present in the soil in unavailable inorganic forms, most of the plants obtain it only as orthrophosphate ions (soluble inorganic forms).However, mycorrhizae, when present, help the plants in obtaining phosphorus. The cycle of phosphorus is schematically represented in Fig. 20.5 and can be well studied two heads:I. Mineralization: Conversion of Organic Phosphorus into Insoluble Inorganic Phosphates
Many soil microorganisms produce enzymes that attack many of the organic phosphorus compounds in the soil and release inorganic phosphate. This process is comparable to the mineralization of organic nitrogen compounds. The enzymes involved in these reactions are collectively called ‘phosphates’ which have a broad range of substrate specificity.
The risk of given the need to administer treatment frequently (because of the inability of immune response to these vectors is negligible. This is an important consideration adenovirus to integrate into chromosomal DNA). They also have advantage that they can accept much larger inserts (up to 35kb).
It is clear that for a wide variety of cell types, adenovirus (Ad) gives more efficient gene transfer compared with other systems, especially in vivo. Ad vectors can transfer genes to both proliferating and quiescent cells. Following delivery, transgene expression is at a high level, but is transient, being low or undetectable in most tissues after two weeks. This is because Ad vectors do not integrate and for safety reasons are disabled for replication.
II. Solubilization: Conversion of Insoluble Inorganic Phosphates into Soluble Phosphates
The availability of phosphorus depends on the degree of Solubilization by various organic and inorganic acids produced by microorganisms in soil. These are the solubilized form of insoluble inorganic phosphates which are taken in by the plants. Fungi, e.g., Aspergillus, Pencillium, Fusarium are the most important of the soil microorganisms which produce substantial amounts of these acids; others are the bacteria, namely, Bacillus, Pseudomonas, Micrococcus, Flavobacterium, etc.
The overall conversion of insoluble inorganic phosphates into soluble inorganic phosphates by the action of acids can be exemplified via reactions as under:
 Schematic representation of Phosphorous cycle
Schematic representation of Phosphorous cycle
The action of acids to convert insoluble phosphates into soluble ones is generally called ‘solubilization’ and particularly takes place in close proximity of the root surfaces where sugar from root-exudates are converted of microorganisms into organic acids.

























0 comments:
Post a Comment