THE
BIOLOGICAL MEMBRANE
INTRODUCTION
Cells
are surrounded by membranes, thin films about 50 Å in width (5-10 nm in
diameter) composed of proteins and lipids, including both glycoproteins and
glycolipids. Intracellular organelles are also compartmentalized by membranes.
Biological membranes are not rigid or impermeable but highly mobile and dynamic
structures. The plasma membrane is the gatekeeper of the cell. It controls not
only the access of inorganic ions, vitamins and nutrients, but also the entry
of drugs and the exit of waste products. Integral transmembrane proteins have
important roles in transporting these molecules through the membrane and often
maintain concentration gradients across the membranes. K+, Na+, and
Ca2+ concentrations
in the cytoplasm are maintained at ~140, 10, and 10-4 mmol/L (546, 23, and
0.0007 mg/dL), respectively, by the transporter proteins, whereas those outside
(in the blood) are ~5, 145, and 1–2 mmol/L (20, 333, and 7–14 mg/dL),
respectively. The driving force for transport of ions and maintenance of ion
gradients is directly or indirectly provided by ATP.
(Figure 1: General overview of Biological membrane)
MEMBRANE LIPIDS
Structure
and properties of membrane lipids
Lipids are nonpolar biomolecules that can be
extracted into organic solvents. They are the major component of fat in adipose
tissue and of membranes in all cells. Fatty acids are common components of both
triglycerides, the storage form of fats, and phospholipids, the major lipids in
cell membranes. Fatty acids in biological systems normally contain an even
number of carbon atoms – a property that stems from their synthesis in
two-carbon units. Long-chain, linear aliphatic C-16 and C-18 fatty acids are
the most common components of phospholipids, and nearly 50% of the fatty acids
in membrane phospholipids are unsaturated, containing one or more carbon-carbon
double bonds. The double bonds in unsaturated fatty acids are all in the cis
configuration. This places a ‘kink’ in their structure and interferes with
their molecular packing, so that lipids enriched in unsaturated fatty acids
have lower melting points (Table 1).
Table
1: Naturally occurring fatty acids
Carbon atoms
|
Chemical formula
|
Systematic name
|
Common name
|
Melting point (°C)
|
|||
Saturated fatty
acids
|
|||||||
12
|
12:0
|
CH3(CH2)10COOH
|
n-dodecanoic
|
lauric
|
44
|
||
14
|
12:0
|
CH3(CH2)12COOH
|
n-tetradecanoic
|
myristic
|
54
|
||
16
|
12:0
|
CH3(CH2)14COOH
|
n-hexadecanoic
|
palmitic
|
63
|
||
18
|
12:0
|
CH3(CH2)16COOH
|
n-octadecanoic
|
stearic
|
70
|
||
20
|
12:0
|
CH3(CH2)18COOH
|
n-eicosanoic
|
arachidic
|
77
|
||
Unsaturated
fatty acids
|
|||||||
Carbon atoms
|
Chemical formula
|
Common name
|
Melting point (°C)
|
||||
16
|
16:1; w-6, D9
|
CH3(CH2)5CH
= CH(CH2)7COOH
|
palmitoleic
|
-0.5
|
|||
18
|
18:1; w-9, D9
|
CH3(CH2)7CH
= CH(CH2)7COOH
|
oleic
|
-13
|
|||
18
|
18:2; w-6, D9,12
|
CH3(CH2)4CH
= CHCH2CH = CH(CH2)7COOH
|
linoleic
|
-5
|
|||
18
|
18:3; w-3, D9,12,15
|
CH3CH2CH = CHCH2CH
= CHCH2CH = CH(CH2)7COOH
|
linolenic
|
-11
|
|||
20
|
20:4; w-6, D5,8,11,14
|
CH3(CH2)4CH
= CHCH2CH = CHCH2CH = CHCH2CH
= CH(CH2)7COOH
|
arachidonic
|
-50
|
|||
Note:
For
unsaturated fatty acids, the ‘w’
designation indicates the location of the first double bond from the methyl end
of the molecule; the D superscripts
indicate the location of the double bonds from the carboxyl end of the
molecule. The melting point of fatty acids, triglycerides and phospholipids
increases with the chain length of the fatty acid and decreases with the number
of its double bonds.
The storage form of lipids is a triacylglycerol (triglyceride)
molecule, with fatty acids esterified to all three of the hydroxyl groups of
glycerol. Both vegetable oils and animal fats are triglycerides, but triolein
(glycerol trioleate, found in olive oil) is a liquid, whereas tristearin
(glycerol tristearate, found in lard) is a solid at room temperature.
Membrane phospholipids are mostly glycerophospholipids, composed
of an L-glycerol backbone with the fatty acids attached at the C-1 and C-2
positions in ester linkage. In general, saturated fatty acids are attached at
the C-1 position, and unsaturated fatty acids at the C-2 position of the
glycerol in phospholipids. Phosphoric acid is linked as an ester to position
C-3, and a polar head group is further linked to the phosphate moiety forming a
phosphate diester bond (Fig. 2). Variations in the size and degree of
unsaturations of the fatty acid components in phospholipids affect the fluidity
of bio-membranes – shorter chain and unsaturated fatty acids decrease the
freezing point of phospholipids, making the membrane more fluid at body
temperature.
Figure 2: Structure of Phospholipid
Phospholipids are amphipathic molecules, because
they are composed of both hydrophobic fatty acids and hydrophilic or polar
head groups. The characteristic head groups of membrane phospholipids are
choline, serine, and ethanolamine (Fig. 3).
When they are hydrated, phospholipids spontaneously form lamellar structures,
and, under suitable conditions, they organize into extended bilayer structures
– not only lamellar structures, but also closed vesicular structures termed
liposomes. Liposomes having defined lipid compositions are being evaluated
clinically for use as drug carrier and delivery systems.
Figure 3: Head groups of
Phospholipid
The liposome is a model for the structure of a
biological membrane, a bilayer of polar lipids with a polar face exposed to the
aqueous environment and the fatty acid side chains buried in the oily,
hydrophobic interior of the membrane. The liposomal surface membrane, like its
component phospholipids, is a somewhat pliant, mobile and flexible structure.
Biological membranes also contain another important amphipathic molecule,
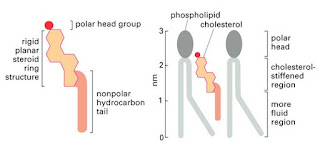
cholesterol, a flat, rigid hydrophobic molecule with a polar hydroxyl group.
Cholesterol is found in all biomembranes and acts as a modulator of membrane fluidity.
At lower temperatures it interferes with fatty acid chain associations and
increases fluidity, and at higher temperatures it tend to limit disorder and
decrease fluidity. Thus, cho-lesterol–phospholipid mixtures have properties
intermediate between the gel and liquid crystalline states of the pure phospholipids;
they form stable, but supple membrane structures (Fig 4).
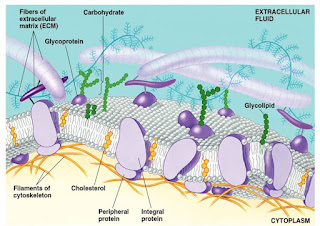
COMPOSITION OF BIOLOGICAL MEMBRANES
Eukaryotic cells have a plasma membrane, as well as
a number of intracellular membranes that define compartments with specialized
functions; differences in both membrane protein and lipid composition
distinguish these organelles. In
addition to the major phospholipids, other important membrane lipids include phosphatidylinositol,
cardiolipin, sphingolipids (sphingomyelin and glycolipids), and cholesterol,
which are described in detail in later chapters.
Cardiolipin
(diphosphatidyl glycerol) is a significant component of the mitochondrial inner
membrane, while sphingomyelin, phosphatidylserine and cholesterol are enriched
in the plasma membrane. The protein to lipid ratio also differs among various
biological membranes, ranging from about 80% (dry weight) lipid in the myelin
sheath that insulates nerve cells, to about 20% lipid in the inner mitochondrial
membrane. Lipids affect the structure of the membrane, the activity of membrane
enzymes and transport systems, and membrane function in processes such as
cellular recognition and signal transduction. Each organelle membrane also has
unique proteins and enzymes that may be used as markers for the purity of
isolated sub-cellular fractions.
Current
structural model of the membrane
The generally accepted model of biomembrane
structure is the fluid mosaic model proposed by Singer & Nicolson in the
early 1970s (Fig. 5). This model
represents the membrane as a fluid-like phospholipid bilayer into which other
lipids and proteins are embedded. As
in liposomes, the polar head groups of the phospholipids are exposed on the
external surface of the membrane, with the fatty acyl chains oriented to the
inside of the membrane. Whereas membrane lipids and proteins easily move on the
membrane surface (lateral diffusion), ‘flip-flop’ movement of lipids between the
outer and inner bilayer leaflets rarely occurs without the aid of an integral
membrane enzyme, flippase (Fig 6).
Although this model is basically correct, there is also growing evidence that
many membrane proteins have limited mobility and are anchored in place by
attachment to cytoskeletal proteins; membrane sub-structures, described as lipid
rafts, also demarcate regions of membranes with specialized composition and
function.
Figure 5: Fluid and Mosaic Model of Biological membrane
Figure 6: Lateral and flip-flop movement of phospholipids
Membrane
proteins are classified as integral (intrinsic) membrane proteins and peripheral
(extrinsic) membrane proteins. The former are embedded deeply in the lipid
bilayer and some of them traverse the membrane several times (transmembrane
protein), whereas peripheral membrane proteins are bound to membrane lipids
and/or integral membrane proteins by noncovalent interactions. Most of the transmembrane segments of
integral membrane proteins form a-helices. They are composed primarily of amino
acid residues with nonpolar side chains – about 20 amino acid residues forming
six to seven a-helical turns are enough to traverse a membrane of 5 nm (50 Å)
thickness. The transmembrane domains interact with one another and with the
hydrophobic tails of the lipid molecules, often forming complex structures,
such as channels involved in ion transport processes.
Biological functions of Membrane
— Maintain a high
concentration of materials in the cell.
— Keep harmful
materials out, Protective barrier.
— Control the
movement of materials into and out of the cell, Semipermiability.
— Let the cell
sense its environment.
— Site of ATP
generation, carry out energy transduction.
— Provide a binding
site for enzymes.
— Modulate signal
transduction.
— Mediate cell-cell
interactions, interlocking surfaces binding cells together (junctions).
— Provide
anchoring sites for filaments of cytoskeleton.
— Assist in reproduction.
Structural
and metabolic role of membranes
A major role of membranes is to maintain the structural
integrity and barrier function of cells and organelles. However, membranes are
not rigid or impermeable: they are fluid, and their components move around, and
they are subject to metabolic turnover. The turnover of membrane components is
especially important for the cellular response to information from inside and
outside the cell: recognition, transfer, amplification, and signal transduction
processes all occur in or on the membranes. Both small and large molecules must
pass through the membrane. With few exceptions, specific membrane proteins
mediate these transport processes.
Phospholipids not only provide a fluid environment,
but also regulate the activities of membrane enzymes. Particular phospholipids
are required for specific membrane structures, such as curved regions and
junctions with adjacent membranes. The inside surface of the membrane is more
suited to phosphatidylethanolamine and phosphatidylserine, in which the polar
heads are small and the hydrocarbons are more spread out, because of their
larger contents of polyunsaturated fatty acids. As a result of such differing
requirements, phospholipids are distributed asymmetrically between outer and
inner leaflets of membranes: phosphatidylcholine and sphingomyelin are more
abundant in the outer leaflet, whereas phosphatidylethanolamine and
phosphatidylserine are enriched in the inner leaflet. Such asymmetries are
actively maintained by flippases, and cell damage often leads to loss of this
membrane lipid asymmetry. Exposure of phosphatidylserine in the outer leaflet
of the erythrocyte plasma membrane increases the cell’s vascular adherence and
is a signal for macrophage recognition and phagocytosis. Both of these
processes probably contribute to the natural process of red cell turnover.
TYPES OF TRANSPORT PROCESSES
Simple
diffusion through the phospholipid bilayer
Small, nonpolar molecules (such as O2, CO2,
N2) and uncharged polar molecules (such as urea, ethanol, and small
organic acids) move through membranes by simple diffusion without the aid of
membrane proteins. The direction of net movement of these species is always
‘downhill’, along the concentration gradient, from high to low concentration to
establish equilibrium.
The hydrophobicity of the molecules is an important
requirement for simple diffusion across the membrane, as the interior of the
phospholipid bilayer is hydrophobic. The rate of transport of a small molecule
is, in fact, closely related to its partition coefficient between oil and water.
Although water molecules can be transported by simple diffusion, channel
proteins are believed to control the movement of water across most membranes,
especially in the kidney for concentration of the urine.
Transport
mediated by membrane proteins
Transport of larger, polar molecules, such as amino acids or
sugars, into a cell requires the involvement of membrane proteins known as
transporters, also called porters, permeases, translocases, or carrier
proteins. The term ‘carrier’ is also applied to ionophores, which move
passively across the membrane together with the bound ion. Transporters are as
specific as are enzymes for their substrates, and work by one of two mechanisms:
facilitated diffusion or active transport.
Facilitated diffusion:
It catalyzes the movement of a substrate through a membrane
down a concentration gradient and does not require energy. In contrast, active
transport is a process in which substrates are transported uphill, against
their concentration gradient. Active transport must be coupled to an energy-producing
reaction.
The
rate of facilitated diffusion is generally much greater than that of simple
diffusion. In contrast to simple diffusion, in which the rate of transport is
directly proportional to the substrate concentration, facilitated diffusion is
a saturable process, characterized by a maximum transport rate, Tmax.
When the concentration of extracellular molecules (transport substrates)
becomes very high, the Tmax is achieved by saturation of the
transporter proteins with substrate. The kinetics of facilitated diffusion for
substrates can be described by the same equations that are used for enzyme
catalysis. The transport process is usually highly specific: each transporter
transports only a single species of molecules or structurally related
compounds. The red blood cell GLUT-1 transporter has a high affinity for
D-glucose, but 10–20 times lower affinity for the related sugars, D-mannose and
D-galactose. The enantiomer L-glucose is not transported; its affinity is more
than 1000 times less than that of the D-form.
Figure 7: Facilitated diffusion with the help of channels
Active transport
Cells may need to move molecules against
concentration gradient. The change in shape of transport membrane transports
solute from one side of membrane to other. It costs energy in the form of ATP.
The proteins involved in transport are also known as protein “pump”. E.g.
Na + / K+ pumps, Ca2+ pump in muscle SER pumps and Proton pump in mitochondria etc.
Figure 8: Sodium Potassium pump action
Transport of large molecules
They move into and out of cell membrane
through vesicles & vacuoles by various mechanism.
Endocytosis
Pinocytosis also known as “cellular drinking” is the most common form of endocytosis. It takes in dissolved molecules as a vesicle. Cell forms
an invagination dissolve in water to be brought into cell. It is non specific
process. Phagocytosis is also
known as“cellular eating”. It used to engulf large particles such as food,
bacteria, etc. into vesicles which are then lead to fuse with lysosomes for
digestion. One more mechanism is Receptor mediated endocytosis. In this process, some integral proteins have receptors on
their surface to recognize & take in hormones, cholesterol, etc into the
cell. These are triggered by molecular signals.
Figure 9: Various
methods of Endocytosis
Exocytosis
The opposite of
endocytosis is exocytosis. Large
molecules that are manufactured in the cell are released through the cell membrane.
Molecules are moved out of the
cell by vesicles that fuse with the plasma membrane. E.g. This is how many hormones are secreted and how nerve cells communicate with one
another.
Figure 10: Mechanism of Exocytosis
References:
Sited from Membranes and Transport (M Maeda)
and the cell (Albert et al)






























1 comments:
One of the greatest info about the cell membrane I ever read.
I also write about the cell membrane you can check it at https://cellmembrane.drreads.com
This article will help me in writing my new article
Post a Comment